Highlights
•
IRBIT is required for homeostasis of the intestinal epithelium
•
IRBIT inhibition of RNR ensures proper intestinal stem cell differentiation
•
Suppression of RNR in intestinal stem cell progeny reverses age-related dysplasia
Summary
The maintenance of the intestinal epithelium is ensured by the controlled proliferation of intestinal stem cells (ISCs) and differentiation of their progeny into various cell types, including enterocytes (ECs) that both mediate nutrient absorption and provide a barrier against pathogens. The signals that regulate transition of proliferative ISCs into differentiated ECs are not fully understood. IRBIT is an evolutionarily conserved protein that regulates ribonucleotide reductase (RNR), an enzyme critical for the generation of DNA precursors. Here, we show that IRBIT expression in ISC progeny within the Drosophila midgut epithelium cells regulates their differentiation via suppression of RNR activity. Disruption of this IRBIT-RNR regulatory circuit causes a premature loss of intestinal tissue integrity. Furthermore, age-related dysplasia can be reversed by suppression of RNR activity in ISC progeny. Collectively, our findings demonstrate a role of the IRBIT-RNR pathway in gut homeostasis.
Graphical Abstract
Subject Areas
Cell Biology
Stem Cells Research
Developmental Biology
Introduction
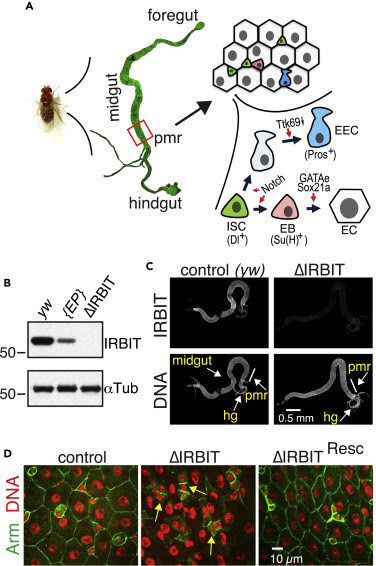
Like the mammalian intestinal epithelium, the Drosophila midgut epithelium is continually renewed by controlled intestinal stem cell (ISC) proliferation and differentiation of their progeny (Micchelli and Perrimon, 2006, Ohlstein and Spradling, 2006). ISC proliferation is finely tuned by diet, aging, and the microbiota ecosystem (Choi et al., 2011, Koehler et al., 2017, O’Brien et al., 2011), using many of the same biochemical pathways that control intestinal epithelial renewal in mammals (Pasco et al., 2015). In addition to self-renewal, ISC division produces two types of postmitotic progeny: enteroendocrine cells (EECs) and enteroblasts (EBs). EBs ultimately mature into adult enterocytes (ECs) (Figure 1A). Mature ECs form the absorptive and protective surface of the epithelium (Micchelli and Perrimon, 2006, O’Brien et al., 2011, Ohlstein and Spradling, 2006, Zhai et al., 2017). Although ISC maintenance and proliferation has been extensively studied, the signals that mediate transition of ISC progeny into terminally differentiated absorptive ECs are not fully understood. The decision of ISC progeny to undergo differentiation is dictated by various intrinsic and extrinsic cues including nutrient availability and the presence of a physical damage in the intestinal epithelium and relies upon the level of interaction between ISC daughter cells. Daughters exhibiting low-level Notch signaling suppress Ttk69 transcriptional repressor and develop into EECs (Beehler-Evans and Micchelli, 2015, Wang et al., 2015, Zeng and Hou, 2015). Daughters with tight connections and strong Notch signaling commit to the EB lineage (O’Brien et al., 2011, Zhai et al., 2017). The process of terminal differentiation of the EB into the absorptive EC is not completely understood but was shown to require the activity of several transcription factors, including Sox21a and GATAe (Zhai et al., 2015, Zhai et al., 2017) (Figure 1A). The delay or block in terminal EC differentiation leads to accumulation of undifferentiated EBs, either causing dysplasia, which can physically damage tissue integrity, or even neoplasia, with mosaic expression of various genes implicated in cancer progression (Chen et al., 2014, Chen et al., 2016, Hsu et al., 2014, Krausova and Korinek, 2014, Zhai et al., 2015).
Ribonucleotide reductase (RNR) is a critical enzyme in the pathway for the de novo dNTP synthesis, as it makes dNDPs from corresponding ribonucleotide precursors via a remarkably complex mechanism (Ahluwalia and Schaaper, 2013, Fairman et al., 2011). RNR consists of two subunits: the R2 subunit provides the free radical that is necessary for R1 subunit-mediated reduction of ribonucleotides. In addition to the catalytic site, R1 subunit has two nucleotide-binding sites that control the state of R1, and one of them, the A-site, monitors R1’s overall activity. RNR is active when ATP binds to the A-site, whereas dATP binding inhibits the enzyme (Ahluwalia and Schaaper, 2013). As the A-site has low affinity for ATP/dATP, the concentrations of dATP required to inhibit RNR usually exceed physiological dATP levels inside dividing cells. We have previously shown that an evolutionarily conserved protein IRBIT (IP3-receptor-binding protein released with inositol 1,4,5-trisphosphate) controls RNR activity by locking the R1 subunit in an R1∗dATP inactive state, in the presence of physiologically relevant dATP concentrations (Arnaoutov and Dasso, 2014). The dNTP pool in HeLa cells is sensitive to IRBIT levels, but the organismal importance of IRBIT-dependent RNR regulation remained unknown, although we speculated that it could control cell-cycle progression and exit (Arnaoutov and Dasso, 2014). High dNTP levels, produced by RNR, are critical for cells to transit through S phase. During Drosophila embryogenesis, the maternal pool of dNTP is only sufficient for the first 10 divisions, after which endogenous RNR activity becomes indispensable (Djabrayan et al., 2019, Song et al., 2017). On the other hand, overexpression of RNR appears to be detrimental for normal progression of embryogenesis (Song et al., 2017), suggesting that there must be mechanisms to curtail RNR activity during cellular specialization. Because dNTP abundance is critical for S phase progression and because the suppression of the cell cycle could regulate differentiation (Djabrayan et al., 2019, Jiang and Kang, 2003, Ruijtenberg and van den Heuvel, 2016, Vastag et al., 2011), we decided to test whether manipulation of the RNR activity could affect cellular decision between proliferation and differentiation.
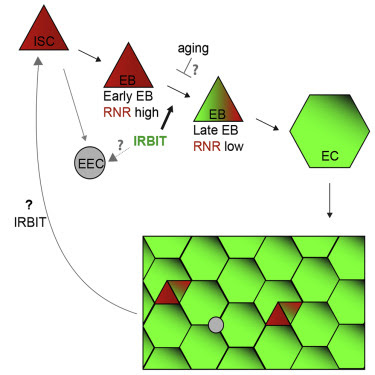
Here, we tested the function of IRBIT in tissue homeostasis, particularly the proliferation and differentiation of ISCs and maintenance of the adult Drosophila midgut epithelium. We found that the IRBIT-RNR pathway is essential to ensure correct differentiation of ISC progeny. We show that conserved transcriptional factor GATAe stimulates IRBIT expression in postmitotic ISC progeny to inhibit RNR and promote differentiation. The intestines of flies lacking IRBIT demonstrate dysplasia, with profound accumulation of undifferentiated ISC progeny. Additionally, we provide evidence that the GATAe-IRBIT-RNR pathway may become dysfunctional as flies age, resulting in characteristic accumulation of undifferentiated ISC progeny. Such dysplasia can be successfully reversed by specifically inhibiting RNR in the ISC progeny. Collectively, these findings show that suppression of RNR activity by IRBIT is an indispensable mechanism that allows the ISC daughter cell to proceed toward differentiation and to maintain intestinal tissue homeostasis.
Results
IRBIT Is Required for Intestinal Epithelial Maintenance
There are two Drosophila genes that encode proteins with significant sequence similarity to vertebrate IRBIT: AhcyL1 (CG9977, IRBIT) and AhcyL2 (CG8956, IRBIT2). Only IRBIT but not IRBIT2 bound RNR efficiently (Figure S1A), suggesting that IRBIT controls RNR in Drosophila. Notably, only IRBIT but not IRBIT2 mRNA was expressed in the midgut during embryogenesis (Figure S1B). Thus, both protein-protein interactions and localized expression prompted us to focus on IRBIT control of RNR regulation in the midgut. We generated two null alleles of IRBIT and we termed flies bearing both as “ΔIRBIT” (Figure S1C). We stained digestive tracts isolated from adult female flies with anti-IRBIT antibodies, confirming IRBIT protein expression in the midgut, as well as its absence in ΔIRBIT flies (Figures 1B and 1C). To verify the specificity of IRBIT loss-of-function phenotypes, we introduced a genomic rescue fragment to a defined docking site in the ΔIRBIT background and termed these flies as “ΔIRBITResc.” ΔIRBIT flies expressed non-functional RNA and lacked IRBIT protein, whereas ΔIRBITResc flies expressed IRBIT RNA at levels similar to controls (Figures S1D and S1E).
We focused on the function of IRBIT in the female posterior midgut region of adult flies (pmr, R5 region [Dutta et al., 2015]) because this tissue has a well-characterized and relatively simple structure (Micchelli and Perrimon, 2006, Miguel-Aliaga et al., 2018, O’Brien et al., 2011, Ohlstein and Spradling, 2006, Zeng et al., 2010, Zeng and Hou, 2015, Zhai et al., 2017) (Figure 1A). By 12 d post-eclosion, the ΔIRBIT pmr epithelium showed degenerate tissue with hyperplastic-like polyps (Figures 1D, S2A, and S2B), which consisted of cells with small nuclei instead of large differentiated ECs. The midguts of ΔIRBITResc flies appeared normal (Figure 1D), confirming that the defects seen in ΔIRBIT midguts result from IRBIT loss. EM ultrastructural analysis revealed that ΔIRBIT midguts have thinner peritrophic membrane (PM), an extracellular matrix barrier against microbial infection (Figure S2C). We examined peritrophic membranes from the midguts of 8-d-old axenic (free from microorganisms) flies by staining with lectin-HPA (Helix pomatia agglutinin). The PMs of ΔIRBIT’s midguts were thinner than PMs of control or ΔIRBITResc flies (Figure S2D), consistent with the EM ultrastructural analysis (Figure S2C). Altogether, our results indicate that the loss of IRBIT in flies leads to formation of intestine that has weak PM and demonstrate tissue dysplasia.
IRBIT Mediates Differentiation of the ISC Progeny
ΔIRBIT midgut dysplasia could result from increased ISC proliferation, failed transition of ISC progeny into EECs and EBs, and/or failed maturation of EBs into ECs. To test these possibilities, we determined the relative abundance of these cell types using specific GAL4 drivers (Figures 2A and S3). esg-Gal4 has a well-defined pattern in the midgut and is expressed in both ISCs and EBs, whereas the expression of Su(H)GBE-Gal4 is restricted to EBs. Antibodies against Delta (Dl) mark ISCs, whereas antibodies against Pros faithfully detect cells of EEC lineage (Zeng et al., 2010). To enhance our arsenal of detection tools, we additionally used antibodies against several proteins that play key functions during the cell cycle and found that antibodies against Asterless (Asl, centriole component) uniformly stain EBs and ISCs, whereas antibodies against Polo (Polo kinase, Plk) preferentially detect ISCs. Antibodies against R1 (RnrL), the large subunit of RNR, revealed that R1 is specifically expressed in both ISCs and EBs (Figure S3). Midguts of ΔIRBIT flies had normal levels of ISCs, increased numbers of the ISC progeny—EBs and immature EECs, and reduced population of ECs. This pattern would be consistent with a block or delay in the differentiation of ISC progeny (Figures 2A, 2B, S4A, and S4B). Clusters of small cells in 8-d-old ΔIRBIT pmr typically consisted of a single ISC and two to four attached undifferentiated progeny (Figure 2C). Moreover, these clusters invariably showed high levels of RNR by immunostaining (Figure 2D), suggesting that ISC progeny in ΔIRBIT midguts expressed high levels of RNR, failed to separate from mother ISCs, and failed to differentiate. By contrast, we did not observe EB groups associated with ISCs in pmr of 8-d-old control flies, indicating that EBs detach from mother ISCs and differentiate rapidly, maintaining normal homeostasis (Figures 2B–2D). Importantly, these R1+ clusters in ΔIRBIT midguts develop rapidly (as early as day 8 post eclosion) because we did not detect significant accumulation of R1+ cells in midguts of newborn ΔIRBIT flies (1 d post eclosion) (Figure S4C).
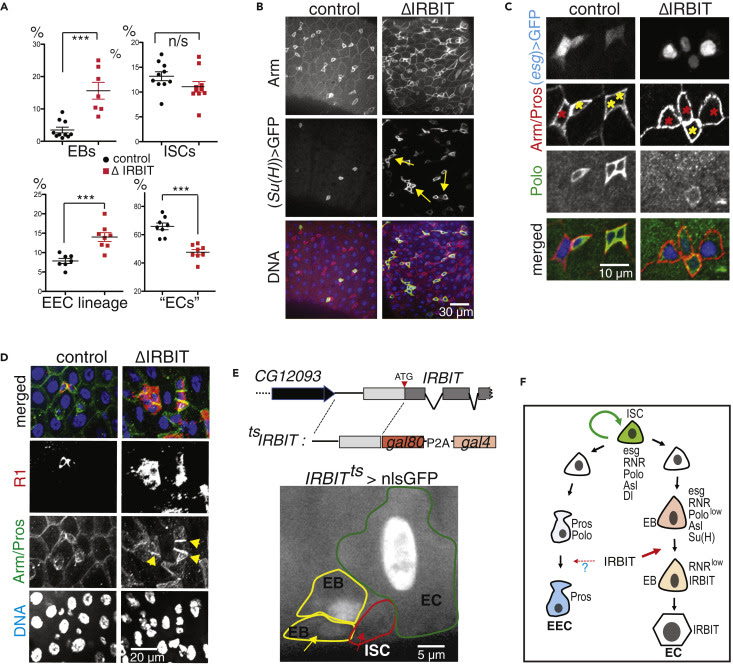
To visualize IRBIT expression during ISC-EB-EC transitions, we used a tsIRBIT promoter (Figure 2E) to express nlsGFP (nuclear GFP) and examined the ISC niche. We found that nlsGFP was not detected in ISCs but accumulated in ISC progeny that were committed to differentiation and remained highly expressed during the EB-EC transition (Figures 2E, S4D–S4F). This pattern suggested activation of IRBIT expression in the differentiating progeny.
Of note, we observed a similar distribution of IRBIT in mouse jejunum (middle part of the small intestine), with high levels in differentiated ECs but low levels in the ISC niche (Haber et al., 2017) (Figure S5), suggesting that IRBIT may play a similar role in mammalian ISC differentiation.
GATAe Stimulates IRBIT Expression to Suppress RNR and to Allow Differentiation of the ISC Progeny
We next tested whether IRBIT expression is controlled by known transcriptional regulators of ISC progeny differentiation (Chen et al., 2016, Zhai et al., 2017). We focused on GATAe, because we noted that during embryogenesis the expressi
…

Leave a Reply