by Liz Ahlberg Touchstone, University of Illinois at Urbana-Champaign
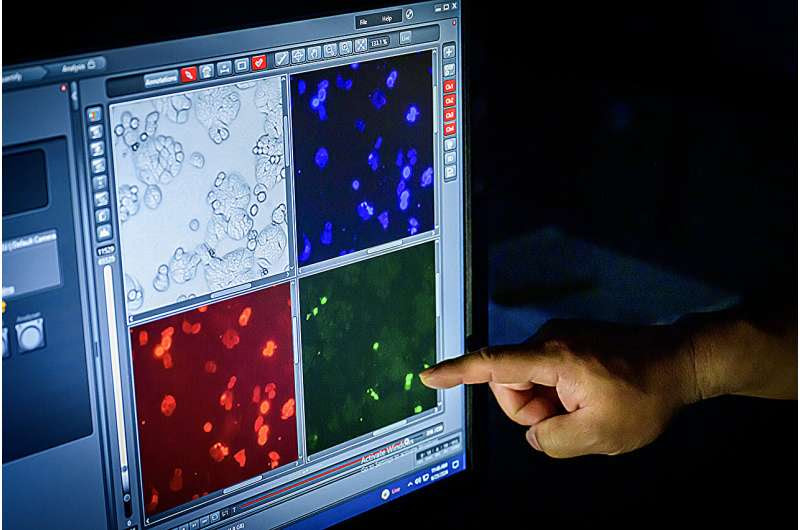
The team uses an inverted fluorescence microscope to image cancer cells that are targeted using different light spectra to initiate inflammatory cell death. The process could help create immunotherapy techniques for disease intervention. Credit: Fred Zwicky, University of Illinois at Urbana-Champaign
A new method of precisely targeting troublesome cells for death using light could unlock new understanding of and treatments for cancer and inflammatory diseases, University of Illinois Urbana-Champaign researchers report.
Inflammatory cell death, knows as necroptosis, is an important regulatory tool in the body’s arsenal against disease. However, in some diseases, the process can go haywire; for example, cancer cells are able to suppress inflammatory signals and thus escape death.
“Usually treatments for cancer use pharmacological induction to kill the cells, but those chemicals tend to diffuse throughout the tissues and it’s hard to contain to a precise location. You get a lot of unwanted effects,” said study leader Kai Zhang, a professor of biochemistry at the U. of I. “We can make the cells responsive to light, and we can focus the light beam to be smaller than one cell. That is how we can use light to very precisely target a cell and turn on its death pathway.”
The researchers use a method called optogenetics to make the cells respond to light. They borrowed a light-activated gene from plants and inserted it into intestinal cell cultures, attaching it to the gene for RIPK3, a protein that regulates necroptosis.
“When activated, RIPK3 undergoes oligomerization—it forms clusters of protein complexes. Our light-sensitive proteins cluster together when exposed to blue light. So by triggering the light-sensitive proteins to come together, the RIPK3 comes together and oligomerizes, and that’s how we mimic the activation pathway,” said graduate student Teak-Jung Oh, the first author of the paper published in the Journal of Molecular Biology.
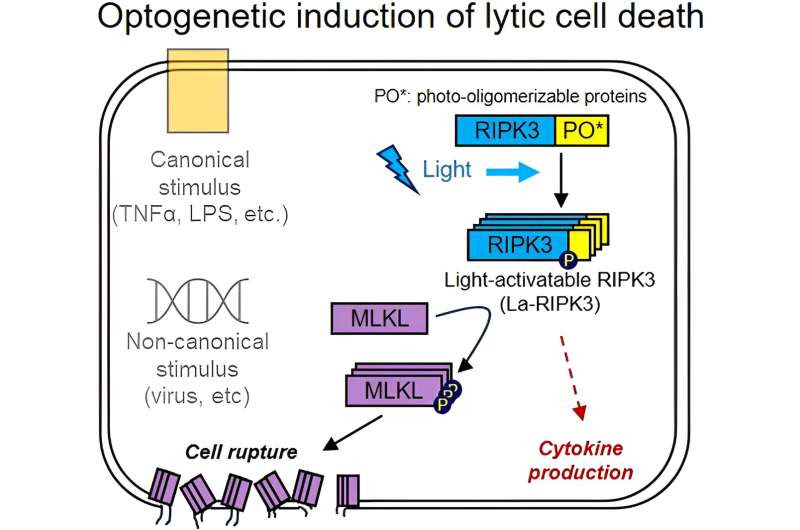
Graphical abstract. Credit: Journal of Molecular Biology (2024). DOI: 10.1016/j.jmb.2024.168628
However, killing the cell itself is not the only goal. Inducing the inflammatory cell death pathway, rather than outright killing the cell mechanically or chemically, triggers the immune system to respond. The ruptured cells release chemicals called cytokines that irritate nearby cells and attract T cells, white blood cells that play an important role in how the immune system identifies and attacks threats, Zhang said.
“Certain cancer cell types create a local immunosuppressive environment, where T cells are either not recruited or, if they do come, they do not recognize it as a threat and do not infiltrate the cancerous area. But by opening up some cancer cells through necroptosis, we hope to modulate this immune suppressive environment and help train the T cells to recognize and attack the cancer,” said Zhang, who is a member of the Cancer Center at Illinois.
Since the optogenetic system requires light delivery directly to tissues, human clinical applications in tissues deeper than skin are currently limited. However, the Illinois group plans to implement their system in mice next to further study necroptosis and immune response in cancer and other inflammatory diseases. They also will further investigate the in vitro platform’s potential for training T cells for immune therapies.
“Understanding the cell signaling pathway for necroptosis is especially important because it has been known to be involved with diseases like neurodegenerative disease and inflammatory bowel disease. Knowing how necroptosis affects progression in these diseases is important. And if you don’t know the molecular mechanisms, you don’t really know what to target to slow the progression,” Oh said.

Leave a Reply