by American College of Physicians
Long-term treatment with immediate-release isradipine does not seem to slow the clinical progression of early-stage Parkinson’s disease. Findings from a multicenter, randomized, parallel-group, double-blind, placebo-controlled trial are published in Annals of Internal Medicine.
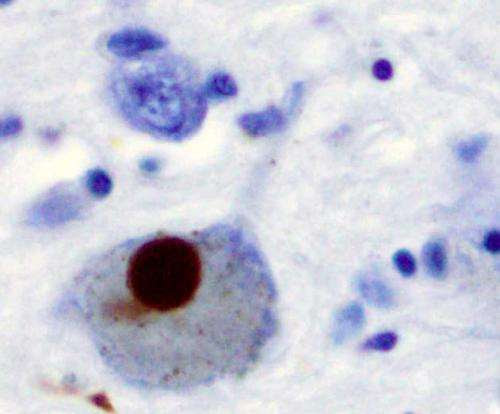
Despite multiple prior studies, there are no proven strategies for slowing the progression of Parkinson’s disease. Isradipine, a dihydropyridine calcium-channel blocker approved for the treatment of hypertension, has been shown to be neuroprotective in animal models Parkinson’s disease. Several epidemiologic studies have demonstrated a reduced risk for Parkinson’s disease in persons receiving dihydropyridines compared with other antihypertensive agents, leading researchers to hypothesize that isradipine may have the potential to slow the progression of the disease when used in its early stages.
Researchers from the Parkinson Study Group STEADY-PD III Investigators team randomly assigned 336 patients with early-stage Parkinson’s disease at 57 Parkinson Study Group sites in North America to either 5 mg of immediate-release isradipine twice daily or placebo for 36 months. None of the participants were taking dopaminergic medications at enrollment. The researchers found no significant difference in the change in Unified Parkinson’s Disease Rating Scale (UPDRS) scores over 36 months in patients receiving isradipine twice daily versus those receiving placebo. None of the secondary outcome measures demonstrated benefit of isradipine.
According to the researchers, these results do not support the hypothesis that isradipine at this dose slows the progression of early-stage Parkinson’s disease.

Leave a Reply