New anti-cancer therapies could simultaneously deplete tumors of energy and boost the body’s immune response against them. (CREDIT: Creative Commons)
Tumor cells are notorious for their adaptability and resilience in the face of treatment, often reshaping their energy metabolism to fuel their rapid growth and invasion. This metabolic shift not only sustains cancer cells but also creates an unfavorable environment for the body’s immune defenses.
In response, a team of researchers led by experts at Massachusetts General Hospital (MGH) embarked on a quest to uncover a potential therapeutic strategy that could simultaneously cripple tumor energy sources while reinvigorating the immune system’s fight against cancer.
In a recent breakthrough study published in Cancer Discovery, Dr. Keith T. Flaherty, Director of Clinical Research at the MGH Cancer Center and a professor of medicine at Harvard Medical School, spearheaded an innovative approach to identify key proteins governing both cancer cell metabolism and immune response within tumors. Their pioneering computational tool, named BipotentR, not only spotted these crucial targets but also offered insights into patient outcomes following immunotherapy.
To unravel the intricate interplay between cancer cell metabolism and immune response, Flaherty and his team developed BipotentR, a cutting-edge computational tool. This tool was designed to pinpoint proteins that could disrupt cancer cell metabolism while simultaneously promoting a robust immune response in the tumor microenvironment.
Utilizing gene expression data from cancer patients who had received immunotherapy, as well as data from cell lines and animal models, BipotentR identified a remarkable 38 cancer cell-specific immune-metabolic regulators.
Artificial intelligence techniques were then employed to assess the activity levels of these regulators within tumors, revealing a fascinating correlation with patient outcomes post-immunotherapy. Among these regulators, one stood out as the top candidate – Estrogen Related Receptor Alpha (ESRRA). Remarkably, ESRRA was found to be highly active in immunotherapy-resistant tumors across various cancer types.
ESRRA: A Double-Edged Sword
The discovery of ESRRA’s pivotal role in cancer therapy marked a significant breakthrough. Inhibiting ESRRA proved to be a potent strategy, as it effectively dismantled tumors by undermining their energy metabolism while simultaneously activating two distinct immune mechanisms involving various immune cell types.
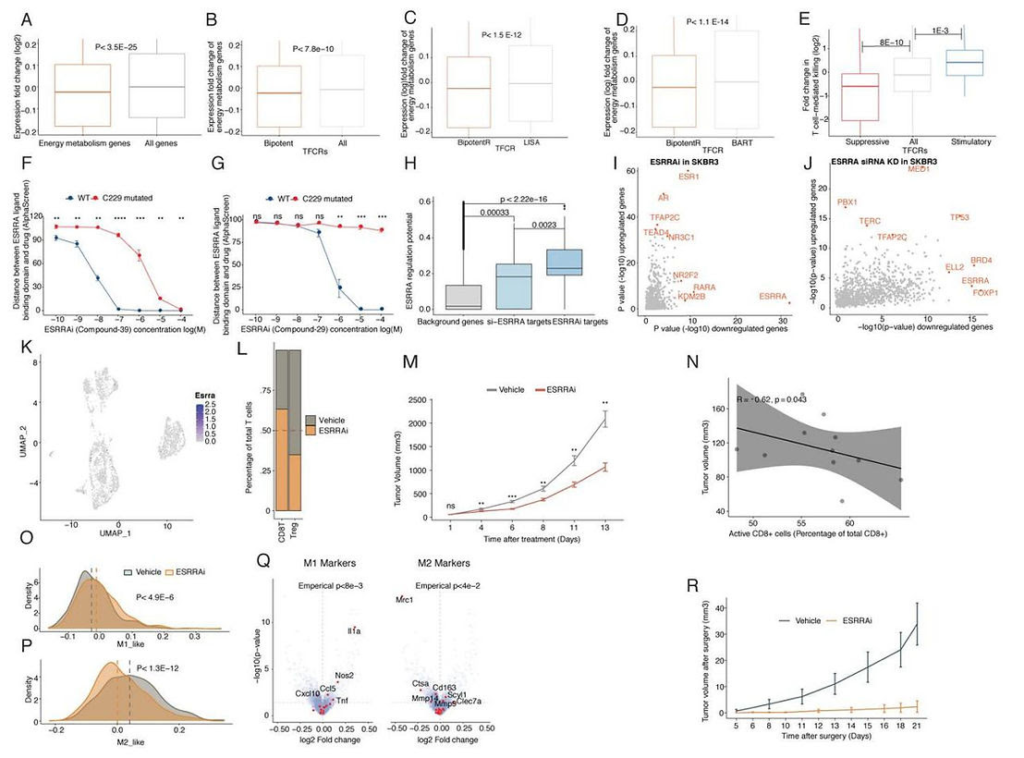
The description and validation of the BipotentR immune module. (CREDIT: Cancer Discovery)
Moreover, the researchers conducted experiments in mice to assess the safety and specificity of ESRRA inhibition. Encouragingly, the effects were focused exclusively on cancer cells, sparing healthy tissue from harm.
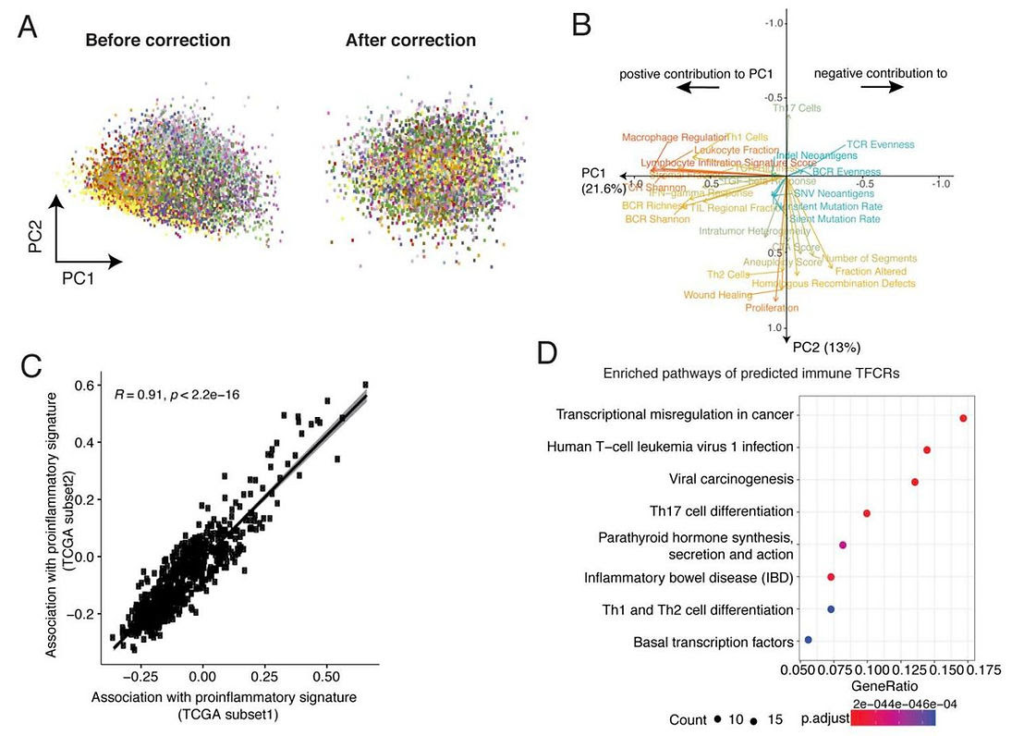
BipotentR: A Versatile Tool for Targeted Therapies
The potential of BipotentR extends beyond ESRRA. The tool can be applied to explore other survival mechanisms employed by cancer cells, such as their ability to stimulate the formation of blood vessels to bolster their nutrient supply. In essence, BipotentR serves as a valuable resource for identifying single drugs that can target specific cancer-related pathways while concurrently stimulating the immune system’s response.
ESRRA inhibition induces antitumor immunity in vivo. (CREDIT: Cancer Discovery)
The implications of this research are profound. BipotentR not only sheds light on novel therapeutic avenues but also offers a straightforward biomarker for predicting patient responses to immunotherapy. By identifying ESRRA as a promising therapeutic target, Flaherty and his team have opened up new possibilities for tailoring cancer treatments to individual patients, increasing the chances of success and minimizing adverse effects.
A Collaborative Effort
This groundbreaking study was a collaborative effort, with additional co-authors from MGH, including Phillip Munson, Dejan Juric, and David E. Fisher. The project received crucial support from the Adelson Medical Research Foundation, underlining the significance of partnerships and funding in advancing scientific discovery.
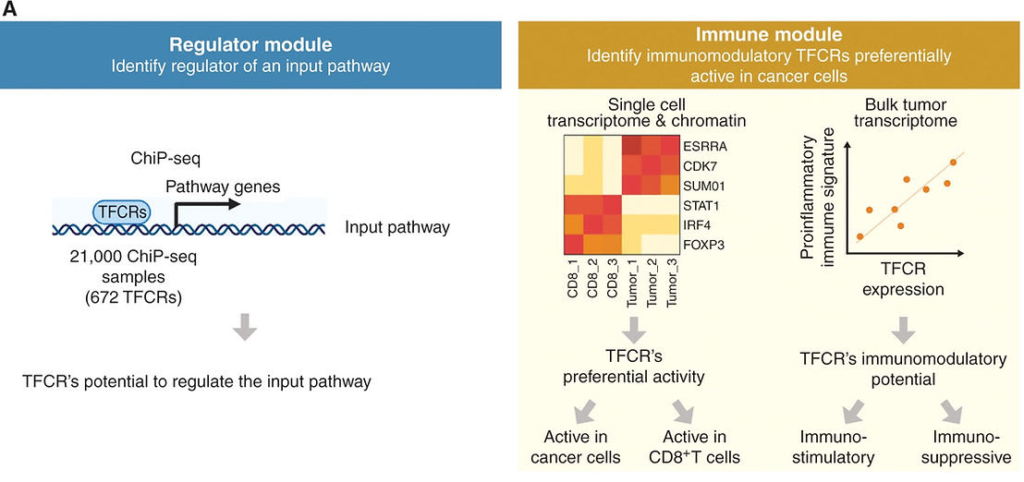
Overall schematic of regulation and immune modules of BipotentR. The regulator module identifies regulators of an input pathway using ChIP-seq data. (CREDIT: Cancer Discovery)
With the development of BipotentR, researchers now have a powerful tool to identify and target specific pathways in cancer cells while harnessing the body’s natural defenses.
This approach not only promises more effective treatments but also offers a means of predicting patient responses, ushering in a new era of personalized cancer care. As we stand on the cusp of these exciting developments, the future of cancer therapy looks brighter than ever.

Leave a Reply