by Tokyo Metropolitan Institute of Medical Science
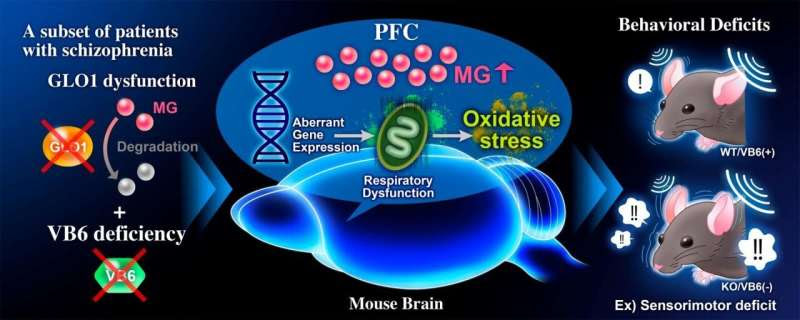
Summary of results. Credit: TMIMS
Methylglyoxal (MG) is a highly reactive α-ketoaldehyde formed endogenously as a byproduct of the glycolytic pathway. MG accumulates under conditions of hyperglycemia, impaired glucose metabolism, or oxidative stress. An excess of MG formation causes mitochondrial impairment and reactive oxygen species (ROS) production that further increases oxidative stress.
It also leads to the formation of advanced glycation end products (AGEs) due to MG reacting with proteins, DNA, and other biomolecules, which can induce aberrant inflammation via binding to receptors for AGEs (RAGE). To remove the toxic MG, various detoxification systems work together in vivo, including the glyoxalase system which enzymatically degrades MG using glyoxalase 1 (GLO1) and GLO2, and the MG scavenging system by vitamin B6 (VB6).
Schizophrenia is a heterogeneous psychiatric disorder characterized by positive symptoms, such as hallucinations and delusions, negative symptoms, such as anhedonia and flat affect, and cognitive impairment. A new study has reported that several patients with schizophrenia have a novel heterozygous frameshift and a single nucleotide variation (SNV) in GLO1 that results in reductions of enzymatic activity. Furthermore, the study reports that VB6 (pyridoxal) levels in peripheral blood of patients with schizophrenia are significantly lower than that of healthy controls. More than 35% of patients with schizophrenia have low levels of VB6 (clinically defined as male: < 6 ng/ml, female: < 4 ng/ml). However, the effects of MG detoxification deficits on the pathophysiology of schizophrenia in vivo remain unclear.
In this study, scientists created a novel mouse model for MG detoxification deficits by feeding Glo1 knockout mice VB6-deficient diets (KO/VB6(-)) and evaluated the effects of impaired MG detoxification systems on brain function. KO/VB6(-) mice accumulated MG in the PFC, hippocampus, and striatum, and displayed behavioral deficits, such as impairments of social interaction and cognitive memory and a sensorimotor deficit in the PPI test. Furthermore, they found aberrant gene expression related to mitochondria function in the PFC of the KO/VB6(-) mice by RNA-sequencing and weighted gene co-expression network analysis (WGCNA). Finally, they demonstrated abnormal mitochondrial respiratory function and subsequently enhanced oxidative stress in the PFC of KO/VB6(-) mice in the PFC. These findings suggest that MG detoxification deficits may cause the observed behavioral deficits via mitochondrial dysfunction and oxidative stress in the PFC.
This is the first report to show that MG detoxification deficits are involved in the development of schizophrenia. Considering the molecular mechanism revealed in this study, antioxidants to prevent oxidative stress and VB6 supplementation may be effective as a new therapeutic strategy for patients with MG detoxification deficits, GLO1 dysfunction, and VB6 deficiency.

Leave a Reply