MAY 31, 2024
by University of Rochester Medical Center
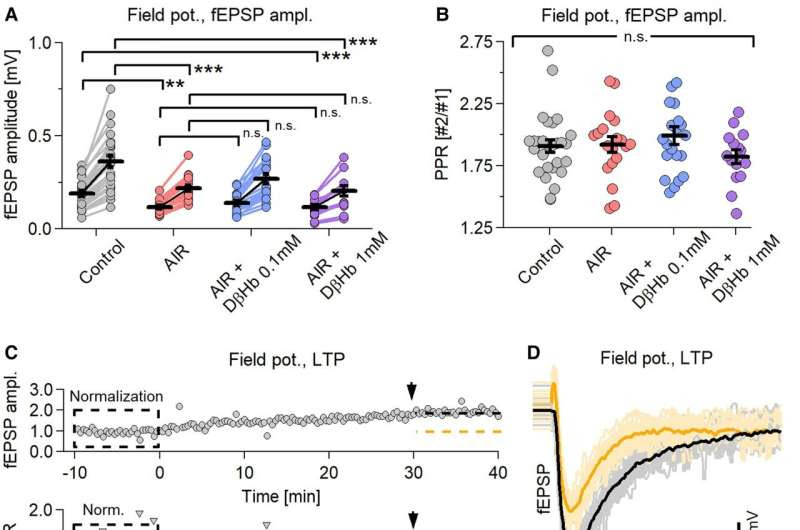
Synaptic activity and LTP, but not neuronal firing or PPR, decrease during paired stimulation under AIR conditions and do not recover during D-ꞵHb administration. Credit: PNAS Nexus (2024). DOI: 10.1093/pnasnexus/pgae196
Researchers at the Del Monte Institute for Neuroscience at the University of Rochester have identified mechanisms in the brain’s hippocampal network that are rescued by ketones. These findings build on previous research showing that ketones can alleviate neurological and cognitive affects.
As we age, our brain naturally becomes more insulin resistant. This creates a breakdown in communication between neurons, causing symptoms like changes in mood, cognitive decline, and eventually neurodegeneration. Nathan A. Smith, MS, Ph.D., associate professor of Neuroscience, and fellow researchers studied the mechanisms in the brain that break down when insulin resistance is suddenly present, like in trauma, but before symptoms manifest into chronic conditions, like diabetes or Alzheimer’s.
“Once neuronal function is lost, there is no recovering the connection, so we need to identify when the function first becomes impaired,” said Smith, the principal investigator of this research, published in the journal PNAS Nexus.
“This study accomplishes that by bringing us closer to understanding how to rescue the function of impaired neurons and prevent or delay devastating diseases like Alzheimer’s.”
Using mice as a model system, researchers focused on the hippocampus, a well understood region of the brain responsible for learning and memory. They found acute insulin resistance impairs several aspects of neuronal function, including synaptic activity, axonal conduction, network synchronization, synaptic plasticity, and action potential properties—the processes critical to support the communication flow in and out of neurons.
Researchers then administered D-βHb, a form of ketones, a byproduct released by the liver when the body burns fat instead of glucose for energy. They found that the synaptic activity that was previously impacted by acute insulin resistance was rescued, conduction in axons increased, neurons were resynchronized, and synaptic plasticity.
“This research has implications for developing ketone-based therapies targeting specific neuronal dysfunctions in conditions involving insulin resistance/hypoglycemia like diabetes or Alzheimer’s disease,” Smith said. “We are now looking to understand the role that astrocytes and other glia cells play in acute insulin resistance.”
Additional authors include Bartosz Kula, Ph.D., of the Del Monte Institute for Neuroscience at the University of Rochester, Botond Antal and Lilianne Mujica-Parodi, Ph.D., of Stony Brook University and Harvard Medical School, Corey Weistuch, Ph.D., of Memorial Sloan Kettering Cancer Center, Florian Gackiere, Ph.D., Alexander Barre, Ph.D., and Jeffrey Hubbard, Ph.D., of Neuroservices Alliance, and Maria Kukley, Ph.D., of Achucarro Basque Center for Neuroscience and Basque Foundation for Science.
More information: Bartosz Kula et al, D-ꞵ-hydroxybutyrate stabilizes hippocampal CA3-CA1 circuit during acute insulin resistance, PNAS Nexus (2024). DOI: 10.1093/pnasnexus/pgae196
Journal information: PNAS Nexus

Leave a Reply