September 5, 2024
by Tokyo Medical and Dental University
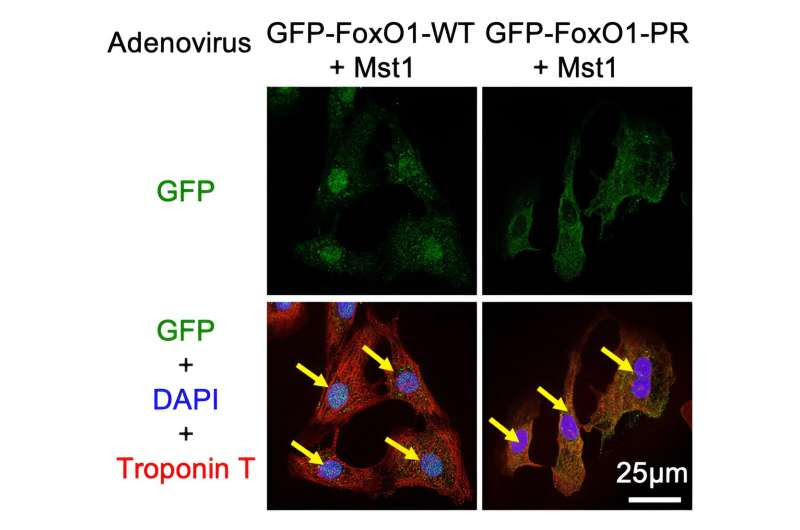
Mst1 causes the transport of FoxO1 from the cell cytoplasm to the nucleus by phosphorylating it. This is shown by the colocalization of the green and the blue stain when Ad-Mst1 is added. Credit: Department of Cardiovascular Medicine, TMDU
Understanding the mechanisms behind cell death and survival is crucial when it comes to conditions like heart failure, which affects millions of people worldwide. Now, researchers from Japan have identified a mechanism which protects cardiac myocytes against ischemia, or a lack of blood supply.
In their study published in Nature Communications, researchers from the Tokyo Medical and Dental University (TMDU) in Japan identified a cellular signaling pathway which stimulates protective mechanisms in cardiac myocytes, potentially opening up avenues for the development of new therapies.
The Forkhead box O (FoxO) family of proteins is involved in many cellular functions, and their cellular activity is tightly controlled. Elaborating further, Dr. Maejima Yasuhiro, the author of the study, says, “The most puzzling aspect of FoxO cellular function is that it regulates both cell death-promoting and -inhibiting mechanisms, even in the same cells.”
Hence, the TMDU researchers focused on the role of FoxOs as well as Mammalian sterile 20-like kinase 1 (Mst1), which is known to interact with FoxOs to regulate processes like cell survival. They found that Mst1 binds to and phosphorylates FoxO1.
Additionally, when both Mst1 and FoxO1 were expressed together in cardiac myocytes, it increased the activity of genes that produce protective antioxidants, while suppressing genes involved in cell death.
But how does this protective mechanism work? To answer this question, the researchers took a closer look at the genes targeted by FoxO1. They found that the antioxidant genes had binding sites for both FoxO1, and another protein called C/EBP-β, while genes involved in cell death had binding sites only for FoxO1.
Subsequently, further experiments showed that in the presence of FoxO1, Mst1 phosphorylated C/EBP-β. This increased FoxO1-C/EBP-β binding, which then stimulated antioxidant production and other pro-survival mechanisms.
What effect does this mechanism have on heart cells? In mice that were genetically engineered to lack FoxO1 or C/EBP-β in the heart, exposure to ischemia for four hours actually leads to an increased amount of dead cardiac tissue.
On the other hand, when mice that lacked FoxO1 were engineered to express a form of phosphorylated C/EBP-β, the amount of dead tissue in the heart decreased. Taken together, these results showed that this Mst1-FoxO1-C/EBP-β interaction protected the heart against ischemia.
In the long term, these findings could pave the way for the development of new treatments for heart failure. “If the level of C/EBP-β phosphorylation can be increased without activation of Mst1, promoting cell survival without activating the detrimental functions of Mst1 may be possible,” explains Prof. Junichi Sadoshima.
In other words, drugs that can selectively promote the protective functions of Mst1 would help protect cardiac myocytes in the case of life-threatening conditions such as heart failure.
Thus, this study not only enhances our understanding of the mechanisms that govern cell death and survival, but also brings new hope for patients suffering from heart failure.
More information: Yasuhiro Maejima et al, Mst1-mediated phosphorylation of FoxO1 and C/EBP-β stimulates cell-protective mechanisms in cardiomyocytes, Nature Communications (2024). DOI: 10.1038/s41467-024-50393-y
Journal information: Nature Communications
Provided by Tokyo Medical and Dental University

Leave a Reply