by Anna Björklund, Identification of a conformational switch in c-MYC.
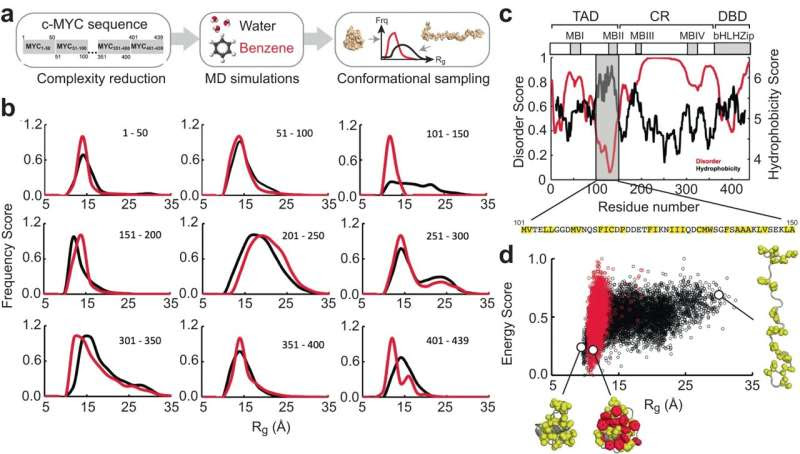
a Overview of the MD strategy employed to identify regions that exhibit probe-induced conformational changes. b Normalized frequency distribution of Rg computed from the ensemble of structures generated from explicit solvent MD simulations in water (black) and water + benzene (red) for all nine c-MYC derived peptides as indicated. c Disorder (VSL2 algorithm) and hydrophobicity (Miyazawa hydrophobicity scale) profile of c-MYC. The modular architecture (TAD: Transactivation Domain, CR: Central Region, DBD: DNA Binding Domain) of c-MYC, MYC Boxes I–IV (MBI – IV) and the bHLHZip domain is shown at the top. The hydrophobic residues between positions 101 and 150 are highlighted in yellow background. d Rg-E (energy normalized in the scale from 0 to 1) distribution plots of coreMYC in water (black) and water + benzene (red) solvents. Representative models of the compact and extended states of coreMYC from the ensemble of MD generated structures are indicated. The hydrophobic residues and the benzene probes are shown as yellow and red spheres, respectively. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-45826-7
In a study published in Nature Communications, KI researchers have revealed a fascinating discovery that could be important in cancer treatments. This new insight involves c-MYC, a protein component that is central to cancer development.
“Our study reveals a chameleon-like segment in the c-MYC protein. This segment alternates between an open and a closed form. In its extended form, the protein is active, and the segment interacts with partner proteins and drives cancer development. But here is the exciting part: By locking this segment in its compact form, we can shut down the functions of the c-MYC protein, offering a new strategy to potentially inhibit cancer development,” says researcher Dilraj Lama at the Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, and first author of the study.
“c-MYC is linked to the majority of cancer types. Its dynamic structure has made it difficult to design drugs that affect protein function,” says Professor Marie Arsenian Henriksson.
“Our breakthrough through the identification of a switch between the active and inactive forms of c-MYC allows us to tame this ‘shape-shifter’ by screening for small molecules that either break binding to partner proteins or lock c-MYC in the inactive form,”
“In a truly interdisciplinary collaboration, we combined computer models with laboratory experiments. We focused on c-MYC, the most well-known cancer protein, and identified its changing structure, says Associate Professor Michael Landreh.
“Through simulations, we localized the transforming region. We then used mass spectrometry and cellular studies to show that a specially designed molecule can lock this region in an inactive form, closing c-MYC’s lines of communication—a crucial step for the development of cancer therapies. This approach can also be translated for other proteins not only to fight cancer, but also to target similar proteins involved in other diseases.”
More information: Dilraj Lama et al, A druggable conformational switch in the c-MYC transactivation domain, Nature Communications (2024). DOI: 10.1038/s41467-024-45826-7
Journal information: Nature Communications
Provided by Identification of a conformational switch in c-MYC.

Leave a Reply