by European Synchrotron Radiation Facility
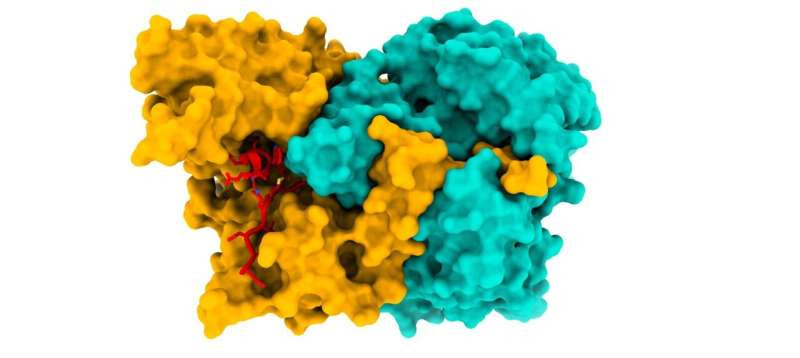
The structure of the ACAD9-ECSIT_CTER complex (ECSIT in red). An international team of researchers, led by the ESRF, have elucidated the structure of the ACAD9-ECSIT_CTER complex, by using cryo-EM techniques. This complex is important in the correct functioning of the energy-producing machinery in mitochondria. Credit: ESRF/M. Soler López. Credit: ESRF/M. Soler López
Researchers led by the ESRF, the European Synchrotron, have found that amyloid oligomers play a role in speeding up mitochondrial energetics during the early stages of Alzheimer’s, in contrast to what has been previously found in more advanced Alzheimer’s brain tissues. The results are published in Nature Communications.
The origin of Alzheimer’s disease, which affects 30 million people worldwide, is still not clear despite an international research effort and significant progress in research.
And yet, identifying the factors driving this incurable neurodegenerative disease is essential to finding better ways to diagnose Alzheimer’s, delay its onset, and prevent progression. “Before understanding the pathology, we need to understand the biology,” explains Montse Soler López, head of the Structural Biology group at the ESRF and co-corresponding author of the study.
Alzheimer’s is an incurable disease that normally appears after the age of 65. However, changes in the brain begin 20 years before the disease appears. “We believe that malfunctioning of the mitochondria can take place 20 years before the person shows symptoms of the disease,” explains Soler López. For a long time, researchers have focused on the amyloid plaques in the brain as the potential cause of the disease. However, this hypothesis is currently being reconsidered.
Now Soler López’s team, together with scientist Irina Gutsche at the Institut de Biologie Structurale (CNRS, CEA, Université Grenoble Alpes) and researchers at the EMBL, conduct a new line of research focusing on aging factors, such as mitochondrial dysfunction. Mitochondria are often referred to as the “powerhouse of cells” because of their essential role in energy production. Over time, mitochondria suffer oxidative stress, and this leads to their malfunction.
A recent finding indicates that individuals with Alzheimer’s may exhibit an accumulation of amyloids within mitochondria, challenging the previous belief that amyloids were solely present outside neurons.
During the initial phases of Alzheimer’s, amyloids typically exist in the form of amyloid-beta oligomers before they turn into fibrils. Soler López and her team investigate what happens within mitochondria during these early stages.
Energy is produced by mitochondria through the respiratory chain, comprised of five protein complexes that must operate cooperatively and effectively for energy generation. Among these complexes, Complex I (CI) holds significant importance as the largest and most critical enzyme in the respiratory chain.
For CI to function properly, assembly factors are essential, and the assembly factor complex known as mitochondrial Complex I Assembly (MCIA), which consists of three core proteins—ECSIT, ACAD9, and NDUFAF1—plays a pivotal role in coordinating CI assembly for optimal functionality.
“We aim to understand the interactions between Complex I and the assembly factor complex during the initial stages of amyloid accumulation, particularly as it unfolds in the pre-symptomatic phases of the disease,” explains Lindsay McGregor, a junior scientist at the ESRF and first author of the paper.
The scientists have determined the structure of the ACAD9-ECSIT complex using cryo-electron microscopy. “This is a breakthrough because knowing the structure enables us to see the insights into the assembly mechanism in detail”, explains Soler López.
Soler López and her colleagues have discovered that ECSIT plays a role in deactivating the fatty acid oxidizing function of ACAD9 through a deflavination process, redirecting the protein to its role in assembling CI. This holds significance for coordinating and regulating cellular energy mechanisms.
Their observations also show that one prerequisite for the proper formation of MCIA is a site-specific dephosphorylation of ECSIT, whereby dephosphorylation enables ACAD9-ECSIT interaction.
Experimental results have revealed that in purified mitochondria, ECSIT dephosphorylation becomes more pronounced in the presence of an accumulation of amyloid-beta oligomers. In turn, this amyloid accumulation results in an overactive CI, in accordance with the increased stability of a dephosphorylated MCIA complex assisting correct CI formation.
These findings diverge from prior studies involving brain tissues from Alzheimer’s patients, which indicated CI shutdown. Soler López explains, “We observe the exact opposite, suggesting that in the early stages of the disease, amyloid-beta oligomers stimulate an overactive CI, creating a detrimental cycle that eventually leads to the impairment of the respiratory chain.”
And she concludes, “These findings offer insights into the role of amyloid-beta oligomers at the disease’s onset, potentially opening new avenues for addressing Alzheimer’s in its early stages.”
More information: Lindsay McGregor et al, The assembly of the Mitochondrial Complex I Assembly complex uncovers a redox pathway coordination, Nature Communications (2023). DOI: 10.1038/s41467-023-43865-0
Journal information: Nature Communications
Provided by European Synchrotron Radiation Facility

Leave a Reply