by University of York
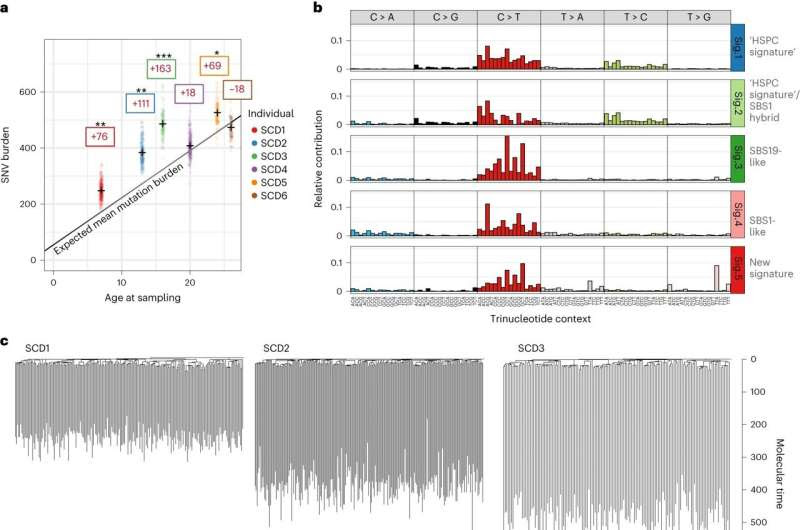
Landscape of somatic mutations in SCD. a, Dot-plot showing the number of mutations per HSPC for each patient plotted against the patient age at the time of sampling. SNV mutation burdens of individual HSPC colonies from before GT, with correction for coverage, are displayed per patient. Mean mutation burdens per individual are indicated by a cross. The black line indicates the expected mean mutation burden by age from a previous study looking at hematopoietically healthy individuals28. The average total number of mutations per HSPC above (+)/below (−) the expected value is indicated in the colored boxes. The mutation burdens for each patient were individually tested against the reference mutation set using a linear mixed-effects model with ‘age’ and ‘patient/reference status’ as fixed effects, and ‘individual’ as a random effect, to see if the ‘patient/reference status’ term was significant (*P < 0.05, **P < 0.01, ***P < 0.001). Exact P values for SCD1 to SCD6 were 9.5 × 10−3, 1.1 × 10−3, 9.7 × 10−5, 0.41, 1.0 × 10−2 and 0.54, respectively. b, Mutational signature analysis reflecting the underlying mutational processes that have been active within sequenced HSPCs. Signatures incorporate the base substitution types in the context of the bases immediately 5′ and 3′ to the mutated bases. Interpretation of each signature, by comparison with known signatures, is shown to the right of each profile. The contributions of each signature to each sample are shown in Extended Data Fig. 2c. Sig., signature. c, Phylogenies showing relatedness of the pre-GT colonies from each individual. Branches are scaled by the number of mutations allocated to that branch and corrected for sequencing depth such that branch lengths reflect the number of mutations acquired in that ancestral lineage. Given the fairly constant rate of mutation acquisition, this is a surrogate for time passed in that lineage and is termed ‘molecular time’. Credit: Nature Medicine (2023). DOI: 10.1038/s41591-023-02636-6
Medical research has shown promising results regarding the potential of gene therapy to cure genetic conditions such as sickle cell disease and the findings of this study, published in Nature Medicine, offer important new insights into processes happening in the body after treatment.
The present study looked at samples from six patients with sickle cell disease who were undergoing gene therapy as part of a major clinical trial at Boston Children’s Hospital. The research brought together an international team of experts, to take a closer look at the genetic changes in the stem cells of patients before and after gene therapy and compare the cells that were modified with the therapy to those that weren’t.
The study highlights the importance of long-term and in-depth monitoring of stem cell samples from patients with genetic conditions to track mutations that could lead to blood cancer, the researchers say.
Co-senior author of the study Professor David Kent from the Department of Biology and York Biomedical Research Institute said, “Think of the gene therapy process like clearing a forest and planting new seedlings. If you imagine that some seedlings are red and some are green, then the ‘selection’ we are observing is akin to having the forest regrow red trees preferentially.”
“Our research indicates that gene therapy imposes a selection on different blood stem cells, the ‘seed’ cells of our blood and immune system. After gene therapy, the treatment might favor growth of stem cells with certain mutations, and this in turn could potentially lead to expansion of blood cells containing these mutations. In other settings, such expansions have been associated with development of blood cancers, particularly in older individuals, but the relationship of this study’s findings to the risk of blood cancers is not yet fully understood.”
The researchers used new technologies in genome science that allow blood cells to be tracked and compared in patients, a new approach which could substantially influence gene therapy trials in the future.
Co-senior author of the study Dr. David Williams from Boston Children’s Hospital and Harvard Medical School noted, “Gene therapy holds immense potential to cure genetic conditions such as sickle cell disease, and understanding how the process influences blood stem cell growth in the long run is crucial for safety.”
“Notably, our study revealed that younger patients, with fewer genetic mutations in their stem cells, didn’t exhibit strong signs of mutations post-therapy. This suggests that treating patients with gene therapy at a younger age could be both safer and more effective, but substantial work needs to be done to test this formally.”
Sickle cell disease, an inherited genetic disorder, alters the natural round and flexible shape of red blood cells into a sickle or crescent shape, leading to severe health issues and putting patients at higher risk of developing blood cancer. Though approximately 100 million people carry the sickle cell trait worldwide, the disease only occurs if a child inherits the trait from both parents. In regions where the trait is prevalent, such as parts of sub-Saharan Africa, up to 20 percent of the population can be affected.
However, recent advances in gene therapy offer hope. This innovative approach involves modifying a patient’s own stem cells outside the body, correcting the faulty gene responsible for the abnormal cell shape. These corrected cells are reintroduced into the patient, aiming to replace the problematic cells with healthy, normal-shaped ones.
The study reveals that the gene therapy treatment itself is not the likely cause of new DNA mutations in blood stem cells. Instead, the process of genetically modifying these stem cells outside the body and re-transplanting them back into the patient makes blood stem cells that already have these mutations more prominent, thereby increasing their influence on the blood and immune systems.
The research team say the findings also suggest that younger patients may have acquired fewer stem cell mutations in their lifetime, which may inform the optimal age for gene therapies in this and other diseases in the future.
Co-lead author of the paper, Dr. Alyssa Cull from the Department of Biology and York Biomedical Research Institute, emphasized the need for further research.
“We now require more in-depth studies to uncover the precise connections behind specific mutations and the gene therapy procedure. There are pressing questions regarding how we can refine gene therapy to avoid stem cells that might contain mutations that affect blood cell growth. Determining whether mutations within a sample of cells are dangerous in the long run remains challenging though and substantial further research is needed.”
Co-lead author Michael Spencer-Chapman from the Wellcome Sanger Institute echoes the need for longer term follow-up of patients saying, “Our research revealed that both gene-therapy modified and unaltered stem cells from sickle cell patients sometimes contained cancer-associated mutations leading to accelerated growth through the gene therapy procedure. Continuously tracking these mutations and gaining a deeper understanding of these processes will profoundly impact the future well-being of sickle cell patients around the world.”
Clonal selection of hematopoietic stem cells after gene therapy for sickle cell disease is published in Nature Medicine
More information: Spencer Chapman, M.et al, Clonal selection of hematopoietic stem cells after gene therapy for sickle cell disease, Nature Medicine (2023). DOI: 10.1038/s41591-023-02636-6. www.nature.com/articles/s41591-023-02636-6
Journal information: Nature Medicine
Provided by University of York

Leave a Reply