by Okinawa Institute of Science and Technology

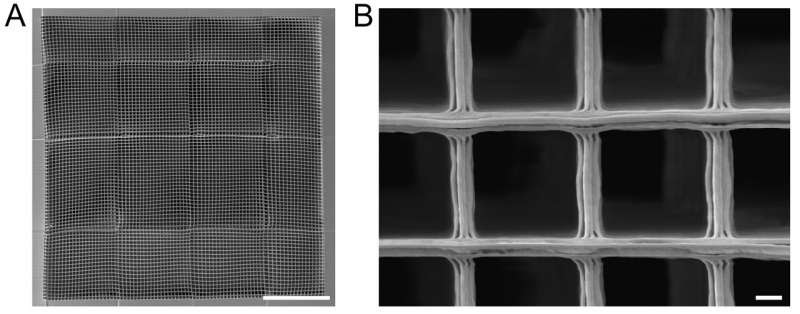
The scaffold, imaged using an electron microscope, is structurally designed to help guide regenerating neurons. Image A: scale bar = 100 µm. Image B: scalebar = 1 µm. Credit: Okinawa Institute of Science and Technology
Across the world, several million people every year suffer from spinal cord injury. These types of injuries break the communication links between the brain and body, reducing movement and sensation, and in the worst cases, can lead to paralysis.
Now, researchers at the Okinawa Institute of Science and Technology Graduate University (OIST) in Japan have used a new technique to create 3D scaffolds that can guide regenerating neurons in the right direction. The scaffolds, which were described in the journal Materials Science and Engineering: C, provide a proof-of-concept that researchers hope could one day could be used to design a structure to help re-connect injured neurons within the human spinal cord.
“Currently, regenerating inured neurons in the spinal cord is a real challenge,” said Professor Marco Terenzio, who leads the Molecular Neuroscience Unit at OIST. He explained that while peripheral nerves, like those in your fingers and legs, can heal themselves relatively easily, most of the neurons in the central nervous system, in the brain and spinal cord, don’t have this level of regenerative potential.
“Only a few types of neurons in the spine have a limited ability to heal,” continued Prof. Terenzio. “And on top of that, the neurons may need to grow up to several millimeters, and there may be scar tissue in the way. So, we need to provide an artificial scaffold to give the neurons a helping hand and bridge the gap.”

The Nanoscribe printing machine uses 2-photon lithography to print and then harden polymer to form specifically designed structures. Credit: Okinawa Institute of Science and Technology
When neurons repair themselves, this process doesn’t occur in isolation. Instead, the neurons rely on an extracellular matrix—a fibrous structure that provides support and chemical cues for the neurons to correctly grow. But so far, technological limitations have prevented scaffolds that can accurately mimic the texture of the extracellular matrix from being manufactured on a large enough scale for spinal cord injury.
In the study, the scientists turned to a state-of-the-art manufacturing technique, called 2-photon lithography, that gave them finer control over the complete structure compared to standard printing methods.
“It works a bit like 3D printing, but in reverse,” explained Prof. Terenzio. “Instead of building up by depositing material where it’s needed, the structure is created by removing material.”
The researchers used computer software to first design scaffolds with grooves and indentations that promoted directional growth of neurons. Neurons typically grow radially, spreading out from a center point, Prof. Terenzio explained, but in injuries that sever a connection, growing in a straight line to bridge the two sides is more efficient. The researchers managed to culture sensory neurons on top of the scaffolds, visualized here using a confocal microscope. Credit: Okinawa Institute of Science and Technology
The researchers then built different scaffolds using a polymer called IP-Dip. This material hardens in response to light from a laser, which is fired at specific positions according to the schematic. The excess, non-hardened polymer was then washed away at the end to reveal the final structure.
When the researchers studied the material properties of the scaffolds, they found that the hardened polymer was thermally and mechanically stable.
The researchers also tested whether the structure was biocompatible, by growing cultured mouse neurons from the dorsal root ganglia—a cluster of neurons that lies close to the spinal cord and relay sensation to the brain. The team also tested the structure with mouse motor neurons, which are found in the spinal cord and are responsible for muscle contraction and consequent movement. Both neuronal types were able to attach and grow over the scaffolds.
The researchers designed one of the scaffolds to be more porous, to encourage the neurons to grow into the structure, as well as over the top.

The neurons successfully grew into scaffold and were imaged using a confocal microscope. The neurons are colored according to their depth in the scaffold. Credit: Okinawa Institute of Science and Technology
“We found that the neurons were able to penetrate all the layers of the scaffold, which was very exciting to see,” said Prof. Terenzio. “The next goal is to use this design as a template for developing future scaffolds that could be used for in vivo experiment in mice.”
The team are also planning to experiment with different materials and scaffold designs that could work better for other types of injuries.
However, the researchers acknowledged, the technology is currently prohibitively expensive to most research labs, and the machine can take days to print scaffolds of sufficient size.
“The technology is still very much in its infancy, but we are hopefully that it will improve in cost and efficiency over time,” added Prof. Terenzio. “We were very fortunate to be able to gain access to this machine through the nanofabrication and mechanical engineering services of the OIST Engineering Section.”

Leave a Reply