July 30, 2024
by Lauren Woods, University of Connecticut
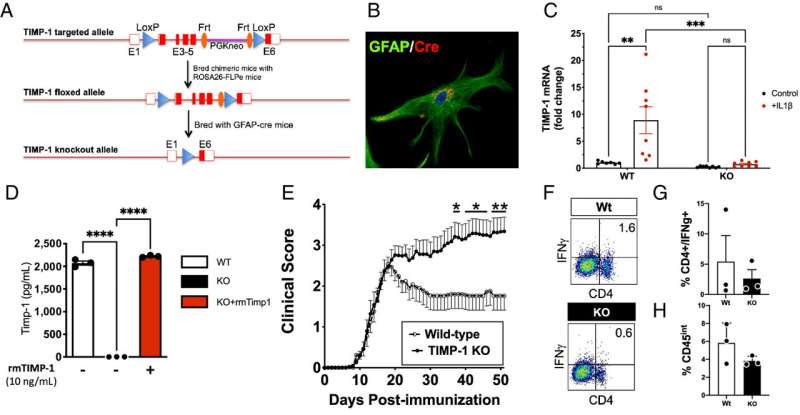
TIMP-1fl/fl knockout mice develop a severe EAE phenotype that is immunologically independent. Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2306816121
with a close family member living with multiple sclerosis Stephen J. Crocker, Ph.D., associate professor of neuroscience and immunology at UConn School of Medicine, takes his research personally.
For the past 15 years as a faculty member in the Department of Neuroscience, he has been involved in both research and advocacy with the National MS Society, annually visiting Washington, D.C. to meet with legislators to advocate for MS patients and research for a cure.
His personal and longtime passion for MS has led to collaborative, innovative multiple sclerosis laboratory research and several breakthroughs, with the latest findings recently published in PNAS. His collaborators are colleagues in UConn Health immunology, cell biology, and neurochemistry, both UConn students and alumni, and researchers from near and far, including Trinity College, and researchers from the Netherlands.
“It has been a great team effort,” says Crocker. To advance MS research, his team has been studying a gene called TIMP-1 that is known to regenerate the tissue in the brain and spinal cord that can be damaged in MS. Their research has focused on a type of cell unique to the brain and spinal cord, called astrocytes, which can make TIMP-1 whenever the brain is injured or under attack.
But in MS patients this protective response of expressing TIMP-1 in the brain can be lost and understanding why this matters is the focus of their study.
To better understand what effect this lack of protein expression has on the brain, the research team leveraged advanced proteomics technology at UConn School of Medicine to decipher the complex mix of factors cells make. This advanced approach allowed them to further assess what exactly astrocytes do when this protein is missing to help develop a “blueprint.”
In their protein analysis, they uncovered a previously known peptide called Anastellin, which had not been known to have a role in MS. They found that Anastellin was overabundant in the brain, and this contributes to the toxic environment inside the brain of MS patients that limits the ability of their brains to naturally repair. Plus, they also discovered that this little peptide blocks an important brain cell receptor needed for a widely used drug to treat MS called Fingolimod to work.
Crocker believes this important research finding may lead to the use of this peptide as future potential biomarker in MS patients to better assess what drug might work best for each individual’s disease, while also allowing for enhanced future drug development to target it.
“It’s a pretty big finding, and it could help ensure MS patients are more rapidly diagnosed and placed on the most effective drug for them, and avoid ones that won’t ultimately work, since we know that the earlier we can treat patients the better off they will be long-term,” says Crocker.
Crocker shares that there are currently no genetic tests for MS, nor are there early biomarkers to identify people who will develop this chronic disease. It also takes a longtime to diagnose MS which is often first identified when people are in their mid-to-late twenties.
“My hope is that we will soon find a new treatment for MS so patients can live their fullest, and most rewarding lives.”
More information: Pearl A. Sutter et al, Astrocytic TIMP-1 regulates production of Anastellin, an inhibitor of oligodendrocyte differentiation and FTY720 responses, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2306816121
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Connecticut

Leave a Reply