By Elizabeth Cooney March 2, 2022 Reprints
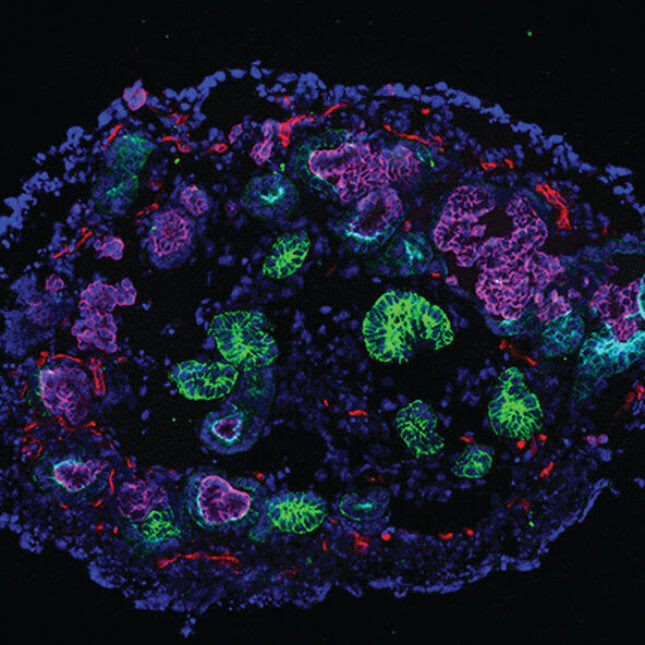
Kidney organoids with markers of nephrons (purple, light blue, green) and stroma (red and yellow). Researchers used the miniature models to study when kidney damage turns irreversible.N. GUPTA, ET AL., SCIENCE TRANSLATIONAL MEDICINE
Chronic kidney disease is a serious medical problem that changes the lives of about 13% of the world’s population. Some kidney damage is reversible; kidney cells can marshal their repair mechanisms to heal harm caused by high blood pressure, diabetes, or harsh medications like chemotherapy. But some damage can become permanent, limiting people’s lives as their kidneys lose their ability to filter blood and remove the body’s waste products.
Just where the tipping point sits between injury that is fixable and damage that’s beyond repair hasn’t been clear.
Now scientists have turned to human kidney organoids, miniature models derived from human stem cells, to determine what marks the point of no return separating reversible and permanent damage. In a new study published Wednesday in Science Translational Medicine, the researchers demonstrated that these organoids are useful models to identify that juncture and they also discovered a drug candidate that could potentially prevent chronic disease before reaching that point.
Navin Gupta, a physician-scientist with a clinical specialty in genetic kidney disease at Massachusetts General Hospital and Harvard Medical School in Boston, talked with STAT about the study he led and where the research might go next. “The whole point of kidney organoid work is for a translational medicine and to impact patient care,” he said. This conversation has been lightly edited for length and clarity.
Give us an idea of the scope of the problem you were trying to solve.
Acute kidney injury is a very, very common clinical condition that often results from different medications. And so here we use human kidney tissue in a test to simulate medication-induced acute kidney injury.
What’s the current treatment?
For many forms of acute injury, the treatment is supportive care, meaning withdrawing whatever is causing the injury, whether it’s blood pressure or certain medications.
What do we already know about how damage progresses?
When the injury is severe enough that there’s scarring and fibrosis of the kidney, that’s irreversible loss of kidney function. By better understanding the transition from an acute injury toward chronic disease, we may be able to understand pathways that help to prevent the loss of kidney function and the development of chronic disease — and foster kidney repair.
What did you set out to do?
To use organoids first to generate a model of human kidney tissue in a dish, and then to use that model to understand the acute kidney injury to chronic kidney disease transition. We leveraged that model to look at mechanisms that would catalyze whether you end up having kidney scarring or a reversible injury where you have preservation of normal kidney architecture.
Why organoids and not, say, lab mice?
There are a lot of pathways in the past that have been looked at with clinical trials based on animal data. And even though many compounds appear safe and effective in animal studies, they don’t translate well to clinical trials in humans.
The whole point of kidney organoid work is for a translational medicine and to impact patient care. And so we’re looking to identify druggable targets and potential existing compounds that show benefit in preventing chronic kidney disease following acute kidney injury.Related:
Listen: A patient and a nephrologist on how using race in kidney testing puts lives at risk
How did you make the organoids?
We take human pluripotent stem cells and we subject them to growth factors and small molecules that simulate kidney development in the human to generate human kidney tissue in a dish.
How did you injure your organoids?
We do know when an injury is either very severe or repeated it’s more likely to cause chronic disease, so we took a very low-level acute kidney injury that we repeated multiple times so that we could monitor for the transition from reversible injury to an irreversible injury. So repeated pulses of cisplatin that simulate chemotherapy regimens that cancer patients receive.
What did you find?
We learned that the DNA damage response in the tubular cells of the kidney helps to determine whether there is going to be recovery versus irreversible damage. When there was that transition from reversible to irreversible disease, it correlated with the loss of homology-directed repair, one of two ways that you repair DNA. That loss of homologous recombination led to the conversion from reversible to irreversible injury in a cisplatin-based model. That was the first observation.
Then we said, is this observation seen in other types of kidney injury? So we interrogated RNA-seq datasets of different common kidney injuries that people do in mice, blocking blood flow to the kidney or tying off a ureter to cause a pressure-related injury in the kidney from the lack of drainage of urine. We wanted to see if the observation we saw in kidney organoids held true in animal models of acute kidney injury and chronic kidney disease. What we found was that in the fibrotic phase, in the irreversible injury phase where kidney tissue is altered beyond repair, that there was a loss of homologous recombination activity in the tubular cells.
Then we did a single nuclear RNA-seq to look at all the homologous recombination genes in the tubular cells and found that indeed they were reduced when there was irreversible kidney damage.
What was your second discovery?
We used small molecule inhibitors to rescue homologous recombination in the damaged tubular cells to show that it preserved kidney structures and reduced fibrosis in the repeated cisplatin model.
What can you tell us about SCR7, the small molecule that worked?
We often use the small molecule to increase homology-directed repair when making site-directed mutagenesis and CRISPR mutants. We do CRISPR genome editing and the standard CRISPR is to just generate a double-stranded DNA break, which knocks out a gene. But sometimes you don’t want to knock out a gene. You want to either knock in a fluorescent signal, or you want to make a site-directed correction if you’re trying to repair the gene. Then you need to increase homology-directed repair, which is sort of a way of seamlessly repairing DNA damage.
So we used a small molecule in the lab previously used to increase homologous recombination. When it seemed like there was an acute drop in homology-directed repair that was the mechanism for the acute kidney injury to chronic kidney disease transition, we did the same injury in the setting of this small molecule known to increase homology-directed repair 19-fold in CRISPR mutants. It rescued homology-directed repair in the tubular epithelial cells, and that ameliorated kidney fibrosis in the kidney organoids.
What happens now?
The next step for this work is to continue to model acute kidney injury and chronic kidney disease in kidney organoids to understand not only this mechanism, but other mechanisms. Obviously, this could translate directly into being used as preclinical studies that could feed in toward clinical trials in human beings.
What we imagine is that kidney organoids can be applied in the preclinical phase of drug discovery to better identify compounds that will be efficacious in humans to reduce the high drug failure in clinical trials.
We imagine ourselves sort of on the cusp of the bench to bedside translation. Our studies are trying to go as close to supporting preclinical studies that the next thing after it would be clinical trials in humans.

Leave a Reply