by Bill Hathaway, Yale University
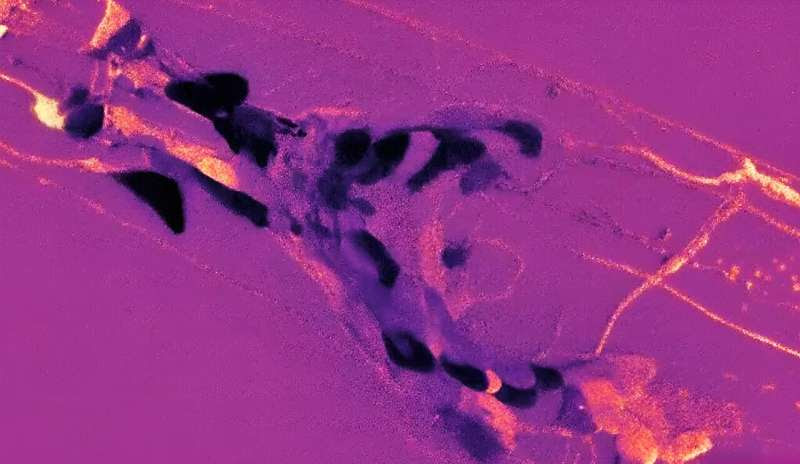
Using a novel biosensor known as HYlight — a fluorescent imaging technology — researchers were able to monitor and map metabolic activity within individual neurons of C. elegans (a type of nematode worm) over time and under different conditions. Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2314699121
Every system in a living organism depends upon a finite supply of energy in order to function. In humans, no organ is more energy-intensive than the brain, which consumes about 20% of the body’s metabolic energy.
But how is energy distributed in the nervous system to assure its functioning? In a new study, Yale scientists unravel part of the mystery. Using a novel biosensor, they were able to map the energy metabolism in single cells of a living organism, the nematode worm C. elegans.
The technology, which was developed by Yale scientists, allowed researchers to map a “landscape” of energy distribution across cells—and even within individual neurons.
Their findings are published in the journal Proceedings of the National Academy of Sciences.
Scientists have long been interested in questions related to the body’s energy metabolism, from how the energy is produced biochemically to how it is distributed throughout the organism—including the brain.
In past studies, neuroimaging technologies such as functional MRI have revealed that energy distribution in the brain changes to accommodate the brain’s different activity states, which underpin thought and cognition. But these technologies lack the cellular resolution needed to understand how energy metabolism is distributed within single cells in the nervous system.
“It’s known that energy production is not distributed equally throughout the brain, but where exactly is it happening? And how does its distribution affect function of the nervous system?” said Aaron Wolfe, a postdoctoral associate in neuroscience at Yale School of Medicine and lead author of the study. “Those were the questions that drove this work.”
To answer them, the Yale team, led by Wolfe and Daniel Colón-Ramos, the Dorys McConnell Duberg Professor of Neuroscience and Cell Biology and co-corresponding author, used a biosensor called HYlight to study metabolic activity within individual neurons in C. elegans under different conditions.
The biosensor was originally developed by a research group led by Richard Goodman, a former faculty member at the Vollum Institute and now an associate research scientist in the Colón-Ramos lab.
The researchers found that energy is differentially distributed across specific cells, and maps onto the identity of individual neurons. This unequal distribution, they said, forms “energy landscapes” that might shape how information flows through neurons and likely influences behavior.
“Energy is the force that animates life. In the context of the nervous system, it animates thought and behavior,” said Colón-Ramos. “Energy is produced via specific metabolic reactions, that we can now track in living animals.
“Visualizing this energy metabolism allows us now to understand how its distribution constrains nervous system function, in health, in disease, and in aging.”
Wolfe added, “By understanding energy production at a cellular level, we can help identify exactly how deficits may arise that can impair neuronal function.”
For example, he said, the researchers found that energy is not only distributed across different cells, but also within compartments of the cells.
“In neurons, our findings indicate that this distribution happen near synapses, which are structures that neurons use to communicate with one another,” Wolfe said. “Maps exist for synaptic connections, and now we can make new maps for energy distribution as animals perform behaviors.”
More information: Aaron D. Wolfe et al, Local and dynamic regulation of neuronal glycolysis in vivo, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2314699121
Journal information: Proceedings of the National Academy of Sciences
Provided by Yale University

Leave a Reply