by University of Birmingham
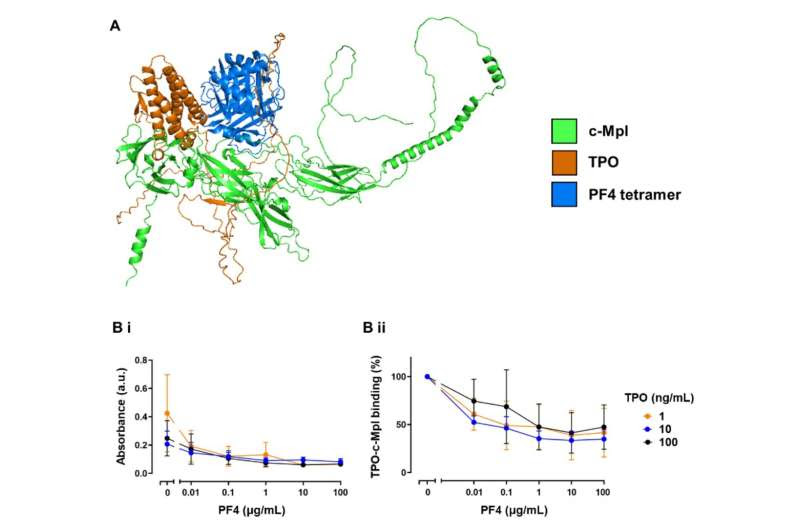
PF4 binding to c-Mpl disrupts TPO binding. (A) AlphaFold prediction of the binding of PF4 and TPO to c-Mpl. Overlayed cartoon structures of c-Mpl (green) complexed with either PF4 (blue) or TPO (orange) as modeled using Colabfold. Structure predictions were ranked by pLDDT, a per-residue measure of the confidence of the predicted structure and the highest ranked were selected. Mean pLDDT for both the c-MplPF4 and c-Mpl-TPO complexes was 69.9. See supplementary methods for further details on interpretation of pLDDT. (B) Competitive binding ELISA. Recombinant c-Mpl was coated onto an ELISA plate then incubated with increasing concentrations of PF4 (0.01 – 100 μg/mL) or vehicle control (PBS). Wells were then incubated with TPO (1, 10, or 100 ng/mL) followed by washing and then detection using an anti-TPO antibody and anti-rabbit secondary HRP. (Bi) absorbance (a.u.) read at 450 nm; (Bii) % change in TPO-c-Mpl binding (n=3). Credit: Blood Journal (2023). DOI: 10.1182/blood.2023020872
A mechanism that led some patients to experience cases of deadly clotting following some types of COVID-19 vaccination has been identified in new research.
In a paper published in Blood Journal, scientists from the University of Birmingham have been able to identify how deadly blood clots, in the disease known as Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT), occur.
Previous studies have shown that patients with VITT produce antibodies that stick to a protein called Platelet factor 4 (PF4) to create a large cluster of molecules called an immune complex. Following the development of a complex, platelets and cells of the immune system causing clotting and inflammation are activated, but the precise nature of what PF4 does in this event was unknown.
In this latest study, the team used blood taken from healthy donors, as well as serum and plasma from patients with VITT, and have been able to learn for the first time how PF4 was directly involved in the activation of platelets and resulted in thrombotic events. By sticking to a receptor called c-Mpl on the surface of platelets, PF4 triggered the production of the small cells known to cause clotting.
Dr. Pip Nicolson, Associate Clinical Professor in Cardiovascular Medicine at the University of Birmingham and senior author of the study said, “The major advances seen in vaccine development during the global COVID-19 pandemic were thrown into sharp relief following the tragic, rare cases of vaccine-induced immune thrombosis.”
“While there were alternative vaccines available to continue to provide protection against the coronavirus in some countries around the world, understanding the mechanisms behind these cases is critical to ensuring that the technology for delivering vaccines can be used with confidence in the future.”
Dr. Richard Buka, Research Fellow in the Institute of Cardiovascular Sciences and co-lead author “As well as identifying a new way in which platelets are being activated in a potentially deadly manner in VITT, our research has also been able to find how this mechanism may lead to new drugs to protect against blood clots in VITT and blood clots in general.”
Variations on a drug used to treat bone marrow cancers could be developed to protect VITT patients from deadly clotting, the research also found.
The team used ruxolitinib, a drug used to treat some types of blood cancer, to block the receptor being triggered by PF4 following the vaccine-induced event. Although they note that the current form of the drug is unsuitable for use in VITT patients, the team nevertheless identified that blocking the pathway through ruxolitinib slowed down platelet aggregation and demonstrates a potential future way to protect patients from blood clots.
Dr. Samantha Montague, Research Fellow in the Institute of Cardiovascular Sciences at the University of Birmingham and co-lead author of the paper said, “It is gratifying that we have been able to identify a new, important biological mechanism through trying to thoroughly understand a new disease. This work helps us to understand more fundamental things about how blood clots form and may also be relevant in other related diseases that are more common.
“Our ongoing research funded by the British Heart Foundation is looking at how we can identify patients who may develop VITT, with a view that future vaccine programmes around the world can be delivered while understanding and managing the potential risk for those few at greatest risk.”
More information: Richard John Buka et al, PF4 activates the c-Mpl-Jak2 pathway in platelets, Blood Journal (2023). DOI: 10.1182/blood.2023020872
Journal information: Blood
Provided by University of Birmingham

Leave a Reply