by The University of Hong Kong
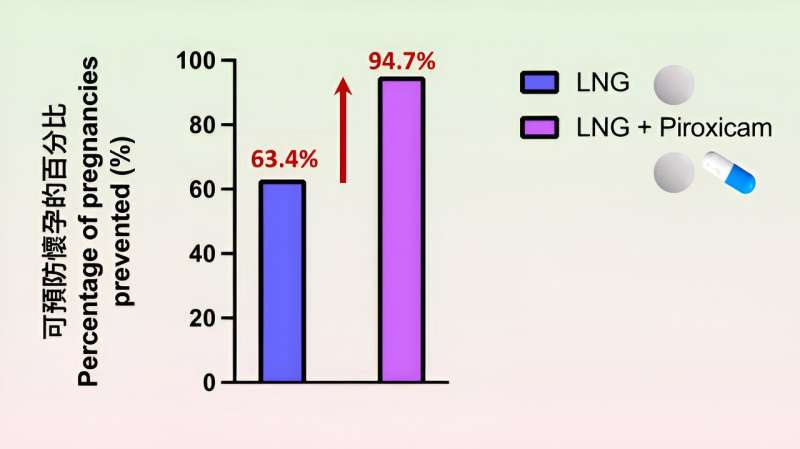
Study shows that the percentage of pregnancies prevented by piroxicam-levonorgestrel co-treatment was significantly higher than that of the levonorgestrel emergency contraceptive pill alone. Credit: The Lancet (2023). DOI: 10.1016/S0140-6736(23)01240-0
A collaborative research team, including members of LKS Faculty of Medicine of the University of Hong Kong (HKUMed), as well as The Family Planning Association of Hong Kong (FPAHK) and Sweden’s Karolinska Institutet, recently published its findings on adding an anti-inflammatory painkiller used for arthritis pain to an oral emergency contraceptive pill (also known as the morning-after pill) to increase the effectiveness of pregnancy prevention.
The study was published in The Lancet.
Emergency contraception is a contraceptive method that can be used to prevent an unintended pregnancy when a regular contraceptive method fails or is not used. It can be in the form of an oral emergency contraceptive pill or the insertion of a copper intrauterine contraceptive device (Cu-IUD).
The oral levonorgestrel emergency contraceptive pill is one of the most popular choices of emergency contraception and is widely used in most countries. It was pioneered by a clinical trial in Hong Kong led by HKUMed’s Professor Ho Pak-chung, published in 1993. However, all contraceptive methods have a failure rate. Hence, the research team is continuing its efforts to explore more effective options.
Prostaglandins are substances produced by most body tissues in humans and mediate a number of biological processes, including inflammatory responses. In the reproductive system, prostaglandin is an important mediator of processes like ovulation, fertilization and embryo implantation. The research team postulated that adding a medication that blocks prostaglandin synthesis may have an additional complimentary effect in achieving contraception.
The research team conducted the first randomized, placebo-controlled trial in the world on the use of piroxicam, a long-acting nonsteroidal anti-inflammatory drug (NSAID) used to treat arthritis pain, which blocks prostaglandin production in the body, in combination with the levonorgestrel emergency contraceptive pill. The findings revealed that with the new combination regimen, only one out of 418 women became pregnant, while seven out of another 418 women receiving levonorgestrel and a placebo became pregnant.
The results showed that the percentage of pregnancies prevented by piroxicam-levonorgestrel co-treatment (94.7%) was significantly higher than that of the levonorgestrel emergency contraceptive pill alone (63.4%). There was no significant difference in the occurrence of adverse effects, including changes to the menstrual bleeding pattern and stomach ache following intake of the two regimens.
Chief investigator Dr. Raymond Li Hang-wun, from HKUMed, said, “Our study is the first to find that piroxicam, a readily available medication, taken at the same time as the levonorgestrel pill can prevent more pregnancies than levonorgestrel alone. We hope these findings will lead to further research and ultimately changes in clinical guidelines to enable women around the world to access more effective emergency contraception.”
Co-investigator Dr. Sue Lo Seen-tsing, from FPAHK, said, “The levonorgestrel pill was registered in Hong Kong in 2002 and has been used safely since then. Locally, emergency contraceptives must be prescribed by health care providers, who assess which emergency contraceptive method is the most suitable in each case.”
“Contraceptive counseling should be provided to help women seeking emergency contraception understand that it cannot replace regular contraceptives and to motivate them to use the latter. Since there is still a small failure rate, a follow-up visit is important. The levonorgestrel emergency contraception pill should be taken within 72 hours after unprotected sex; the earlier it is taken, the more effective it is.”
More information: Raymond Hang Wun Li et al, Oral emergency contraception with levonorgestrel plus piroxicam: a randomised double-blind placebo-controlled trial, The Lancet (2023). DOI: 10.1016/S0140-6736(23)01240-0
Journal information: The Lancet
Provided by The University of Hong Kong

Leave a Reply