by Ruhr-Universitaet-Bochum
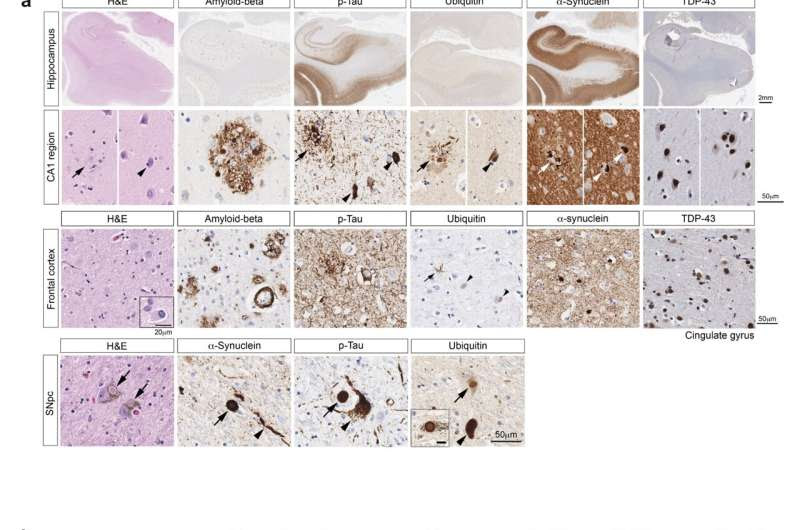
NEMO is associated with pathological protein aggregates. Credit: Nature Communications (2023). DOI:10.1038/s41467-023-44033-0
Researchers have identified a mechanism that promotes the breakdown of harmful protein deposits. If it malfunctions, it can lead to Parkinson’s disease.
NEMO, a protein that is primarily associated with signaling processes in the immune system, prevents the deposition of protein aggregates that occur in Parkinson’s disease. For this purpose, it binds to certain protein chains that serve as markers for cellular waste removal, thus promoting the degradation of the harmful aggregates.
A research team headed by Professor Konstanze Winklhofer from Ruhr University Bochum, Germany, has shed light on how this mechanism works.
The team published their findings in the journal Nature Communications. In follow-up studies, the team is investigating ways to harness the findings for therapeutic strategies.
Looking for targeted therapeutic approaches
Neurodegenerative diseases, such as Parkinson’s or Alzheimer’s disease, are associated with the deposition of protein aggregates in the brain. These aggregates accumulate when the cellular waste removal system is defective or overloaded. In Parkinson’s disease, aggregates consisting primarily of the protein ⍺-synuclein are found in certain regions of the brain.
“The fact that such aggregates occur, which are referred to as Lewy bodies, is a key feature of Parkinson’s disease,” explains Winklhofer.
The misfolding and aggregation of ⍺-synuclein is of crucial importance for processes that lead to the loss of function and death of neuronal cells and contribute to the progression of the disease. Researchers from various disciplines around the world are therefore aiming to decipher these processes at a cellular and molecular level, in order to develop targeted therapeutic approaches.
Labeled proteins are degraded
In collaboration with an interdisciplinary team, the Bochum-based research group has succeeded in gaining a better understanding of the mechanism of ⍺-synuclein degradation: Cellular proteins that are destined for degradation are labeled so that the cellular waste disposal system can identify these proteins. This label consists of a chain of ubiquitin molecules.
“Depending on how these ubiquitin molecules are linked and the length and structure of the ubiquitin chains, the cellular waste disposal system can recognize through which pathway the labeled proteins shall be degraded,” says Winklhofer.
The protective effect of NEMO protein
In previous studies, the research group has shown that a specific form of ubiquitin chains, so-called linear ubiquitin chains, accumulates on protein aggregates in neuronal cells and reduce the toxicity of the protein aggregates. The researchers have now finally identified the mechanism of this protective effect. They found that the protein NEMO docks to linear ubiquitin chains on the protein aggregates and promotes the degradation of ⍺-synuclein.
“What’s interesting is that the protective effect of NEMO can be blocked by inhibiting autophagy,” explains Winklhofer. Autophagy is an important component of cellular waste removal, and it means that the material that needs to be degraded is packed into membrane-enclosed vesicles, which then fuse with lysosomes. Lysosomes are small cell organelles that contain various enzymes for the degradation of biomolecules.
The research group of Konstanze Winklhofer discovered that NEMO interacts with a protein of the autophagy machinery. By forming a complex consisting of linear ubiquitin chains, of NEMO and the said protein called p62 on the ⍺-synuclein aggregates, p62 can be arranged in the form of condensates—which is essential for the efficient recruitment of the autophagy machinery to protein aggregates.
Mutation in the NEMO gene results in early and severe disease
“A milestone during this research was a conversation with neurologists from the University of San Francisco, who had contacted me about an interesting case,” says Winklhofer. Her US colleagues were treating a patient with progressive Parkinson’s disease who had fallen ill in her early 40s. They therefore ran a genetic test, which revealed that this patient had a rare mutation in the NEMO gene.
“Our biochemical and cell biological characterization of the NEMO variant showed that it’s unable to bind to linear ubiquitin chains and therefore can’t dock to protein aggregates.” The loss of function of NEMO impairs the formation of p62 condensates on ⍺-synuclein aggregates and disrupts their degradation.
In fact, a pronounced deposition of ⍺-synuclein aggregates was found in the brain of the patient with the NEMO mutation. “It should be noted that other protein aggregates could also be detected, such as those that occur in Alzheimer’s disease,” says Winklhofer. “This explains the severe course of the NEMO-associated disease and underpins a general role of NEMO in the quality control of aggregated proteins.”
The research team is conducting follow-up studies to explore the potential of NEMO and linear ubiquitin chains for new therapeutic strategies.
More information: Furthmann, N., Bader, V., Angersbach, L. et al, NEMO reshapes the α-Synuclein aggregate interface and acts as an autophagy adapter by co-condensation with p62. Nature Communications (2023). DOI: 10.1038/s41467-023-44033-0
Journal information: Nature Communications
Provided by Ruhr-Universitaet-Bochum

Leave a Reply