by University of Barcelona
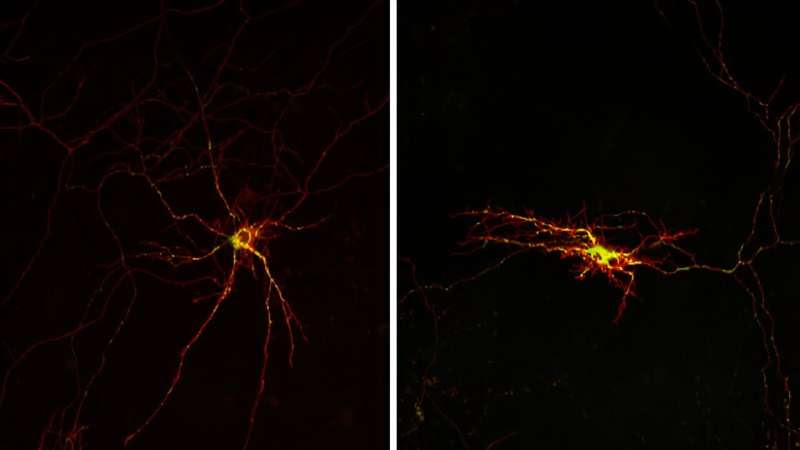
The study led by the UB, IBMB-CSI, and CIBERNED reveals a decisive mechanism related to neuronal death and motor alterations in the most evolved mammals, which could help fight neurodegenerative diseases. Credit: Science Signaling (2024). DOI: 10.1126/scisignal.abq1007
The human brain is an organ that requires 20% to 25% of the energy the body creates. This high energy demand for neuronal functions depends on the transport and precise distribution of mitochondria—the energy-generating cell organelles—in each neuron.
Now, a study published in the journal Science Signaling has identified, for the first time, a molecular complex that affects transport of mitochondria within neurons and regulates neuronal death.
The discovery of the complex, exclusively present in the most evolved mammals, could help to locate new therapeutic targets against neurodegenerative diseases such as Parkinson’s, neuromuscular diseases, or even some types of tumors.
The study, conducted on animal models and cell cultures, was led by Professor Eduardo Soriano, from the University of Barcelona and the Institute of Neurosciences of the UB (UBneuro), and the Biomedical Research Networking Center on Neurodegenerative Diseases (CIBERNED), and researcher Anna María Aragay, member of the Spanish National Research Council (CSIC) and the Institute of Molecular Biology of Barcelona (IBMB-CSIC).
Bringing energy for neuronal functions
“In neurons, the transport process of mitochondria is determining, since these organelles must be present along all axons and dendrites—neuron extensions—to provide energy to the neurotransmission and the neuronal functions, processes that require a lot of energy. This great consumption depends on a specific and precise distribution of mitochondria within neurons,” notes Soriano, co-director of the study and member of the Department of Cell Biology, Physiology and Immunology at the UB’s Faculty of Biology.
The study reveals the Alex3/Gαq mitochondrial complex interacts with the mitochondria machinery to distribute and transport these cell organelles along the neurons’ axons and dendrite. This process depends on the interaction of the Gq protein with the Alex3 mitochondrial protein.
“For the first time, we found that the Alex3/Gαq is essential not only for the transport and mitochondrial function, but also for neuronal physiology, movement control and neuronal viability. If this system is inactivated—for instance, in mice with a specific deficiency of the Alex3 protein in the central nervous system—the mitochondrial trafficking is reduced, there is less dendritic and axonal arborizations and this causes motor deficits and even neuronal death,” says Aragay, co-director of the study.
The authors of the study had previously described in other articles that the Alex3 and Gαq proteins regulated mitochondrial transport. However, they did not know how these interacted or what molecular mechanisms took part in the process.
The interaction of the Alex3/Gαq mitochondrial complex is regulated through the G protein-coupled receptors (GPCR), according to the study. These receptors have many molecules—neurotransmitters, hormones, cannabinoids, etc.—with different functions in the organism.
“The activation of GPCRs not only alters the mitochondrial distribution but also its function, and as a notable effect, the neuronal growth and viability. Our study suggests that, in general, these molecules that interact with these receptors could regulate several aspects of the mitochondrial biology through the GPCR,” note the experts.
More information: Ismael Izquierdo-Villalba et al, A mammalian-specific Alex3/Gα q protein complex regulates mitochondrial trafficking, dendritic complexity, and neuronal survival, Science Signaling (2024). DOI: 10.1126/scisignal.abq1007
Provided by University of Barcelona

Leave a Reply