by The Francis Crick Institute
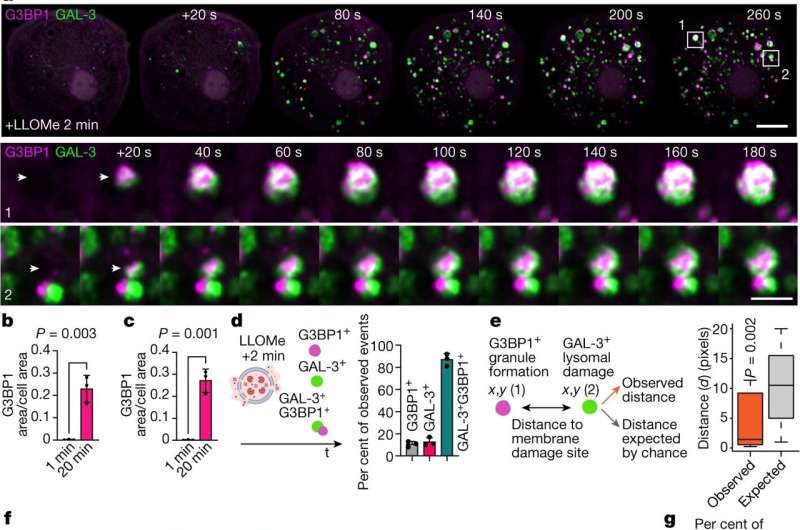
Stress granules form in the proximity of endomembrane damage sites. a, Live-cell imaging sequence of iPSDMs expressing G3BP1–GFP and GAL-3–RFP after 2 min of LLOMe treatment (1 mM). Outlined regions in the top row are magnified in the bottom two rows. Arrowheads highlight the time frame before and after a G3BP1/GAL-3 event is detected. b,c, Quantification of area of G3BP1 (b) and GAL-3 (c) puncta normalized to cell area from live-cell imaging experiments as described in a. n = 30 cells examined over 3 independent experiments; two-tailed t-test. d, Scheme summarizing the different type of events observed in the first 20 min after inducing lysosomal damage. Graph shows the percentage of single G3BP1+ events, GAL-3+ events or combined GAL-3+G3BP1+ events observed by live-cell imaging. e, Spatial point pattern analysis applied to G3BP1+ and GAL-3+ events. In box plots, the centre line is the median, boxes delineate the interquartile range (IQR), and whiskers represent the data range within 1.5 times the IQR. n = 40 events examined over 3 independent experiments; P value by Monte Carlo simulation-based approach. f, G3BP1 polarized ‘plug pattern’ on GAL-3+ damaged vesicles and the corresponding 3D z-stack reconstruction. g, Quantification and schematic of plugging events shown in f. n = 40 events examined over 3 independent experiments. SG, stress granule. h, Image sequence (1 s frame rate, shown at 5s intervals) of iPSDMs expressing G3BP1–GFP (magenta) and GAL-3–RFP (green) after 2 min of LLOMe treatment. i, Mean fluorescence intensity (MFI) over time of G3BP1+ and GAL-3+ events as shown in h. Graphs show traces over time and standard error. n = 30 events examined over 3 independent experiments. AU, arbitrary units. j, Live-cell imaging sequence of iPSDMs expressing LAMP1–RFP and G3BP1–GFP. Arrowhead indicates membrane rupture site. The panel on the far right shows a 3D reconstruction. k, MFI of G3BP1–GFP over time for sequence shown in j. Bar plots indicate mean ± s.e.m. of at least three independent experiments. Scale bars: 10 μm (a, top), 2 μm (a, middle, bottom, f,h,j). Credit: Nature (2023). DOI: 10.1038/s41586-023-06726-w
Researchers at the Francis Crick Institute have found that cellular structures called stress granules perform an essential protective function in support of the immune response against infections like tuberculosis (TB).
This biological insight may also help researchers understand the role of similar organelles in protecting against diseases that also cause cell damage, including cancer and neurodegeneration.
During infection with Mycobacterium tuberculosis, immune cells called macrophages are recruited to envelop the bacteria and remove the threat. But the bacteria can secrete chemicals to damage the macrophage membrane and escape their ‘immune prison’.
The team’s results, published today in Nature, show that when macrophage membranes are ruptured, stress granules rapidly form a plaque to plug the gaps, allowing for cellular repair machinery to come and fix the damage.
The team also showed that the ability to recruit these “molecular plasters” was essential to keep infection under control. When they edited infected cells to remove genes responsible for stress granule formation, macrophages could no longer envelop and destroy bacteria, allowing the infection to take over.
Stress granules form a plug to repair damaged lysosome membranes inside macrophages. Credit: Claudio Bussi.
This plugging observed in macrophages was confirmed using artificial vesicles formed in vitro in a collaboration with Agustin Mangiarotti in Rumiana Dimova’s Lab at the Max Planck Institute of Colloids and Interfaces, Potsdam, Germany.
In collaboration with Christian Vanhille-Campos at the group headed by Anđela Šarić at the Institute of Science and Technology in Vienna, Austria, they also validated their results from the lab with computer models of organelle damage and repair, which showed similar patterns of plaque formation over areas of damage.
Claudio Bussi, Postdoctoral Fellow at the Crick and first and co-senior author on the study, said, “Antibiotic resistance presents a very real threat to the control of infections like TB, so it’s essential that we understand how the body responds to the challenge of infection.
“Not only have we uncovered a new protective role for stress granules, but demonstrated how essential they are in supporting the immune response against TB.”
The team plan to continue studying the role of stress granule and possibly other similar molecules in order to find ways of boosting natural defenses against infection.
Max Gutierrez, head of the Host-Pathogen Interactions in Tuberculosis Laboratory that supervised the study at the Crick, said, “There are a number of different membraneless molecules, like stress granules, many with unknown functions. It’s possible that they may have a role as plasters or scaffolds, helping protect the integrity of cells that undergo injury.”
“This is not only important in infection, but also other diseases where cell and organelle damage can occur, like cancer and neurodegenerative diseases like Parkinson’s disease.”
More information: Claudio Bussi et al, Stress granules plug and stabilize damaged endolysosomal membranes, Nature (2023). DOI: 10.1038/s41586-023-06726-w
Provided by The Francis Crick Institute

Leave a Reply