According to their website, Rexgenero is a “regenerative medicine company and developer of advanced cell-based therapeutics for the treatment of serious diseases that are poorly treated with existing therapies.” The head office of Rexgenero is in London, but their Research and Development laboratories are in Seville, Spain. They also have a United Kingdom office in Brighton, which is a town with which I have some familiarity, since I lived there at one time in my life.
Rexgenero has focused on developing regenerative products from bone marrow cells. In particular, they have used bone marrow-derived mononuclear cells (BM-MNCs). BM-MNCs are extracted from the patient’s bone marrow after a bone marrow aspiration. To isolate the mononuclear fraction, the white blood cells fraction from bone marrow is usually isolated by centrifugation with a material called Ficoll. The pelleted material is the mononuclear fraction and contains a potent mixture of lymphocytes, monocytes, mesenchymal stem cells, hematopoietic progenitor cells, and other less well-characterized cell populations.
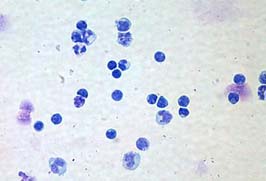
Another component of bone marrow mononuclear cells is endothelial progenitor cells (EPCs), which give rise to the walls of blood vessels. When locally infused locally at the site of diseased vascular tissue, a well-processed mononuclear fraction can potentially form new blood vessels and restore tissue that was formerly starved for oxygen (see Haider, KH, Aziz S, and Al-Reshidi MA, Regenerative Medicine, 2017 12(8):969-982 for a review).
A bone marrow-based product developed by Rexgenero, called REX-001, has been used in human clinical trials to revascularize patients with a rather nasty condition called Critical Limb Ischemia (CLI). CLI occurs when the blood vessels that feed and nourish an arm or leg are severely damaged or obstructed. This results markedly reduces blood flow to the limb and the oxygen-deprived tissues can begin to die off, which causes excruciating pain, and disfiguring skin ulcers or sores.
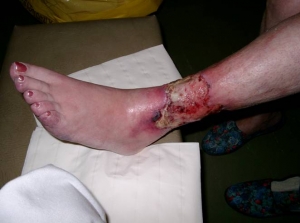
These ulcers can become infected, gangrenous, and may require debridement or, in the most severe cases, limb amputation (yikes!).
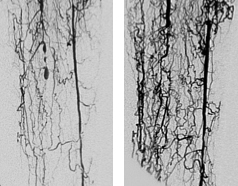
Can REX-001 administration treat patients with CLI? In an earlier Phase II clinical trial, which was completed in 2016, REX-001 was administered to over 100 patients in three clinical trials. The results were rather encouraging. Patients who had severe ischemic pain at rest without skin ulcers (Rutherford scale in category 4), or resting pain with skin ulcers (category 5) were treated with REX-001 for 12 months. At the end of 12 months, most patients (the website does not say what percentage of patients) in both patient populations were devoid of CLI and showed significant improvement in their clinical condition, as assessed by changes in Rutherford category. Patients showed complete ulcer healing and alleviation of rest pain. Angiographic imaging of treat patients definitively showed that REX-001-treated patients had extensive new networks that blood vessels that had sprouted. It is reasonable, in my view to suspect that these new blood vessel networks in the limb were responsible for the corresponding improvement in patient’s clinical condition.
Rexgenero has recently announced the treatment of its first patient in a Phase III clinical trial that will evaluate REX-001, in patients with CLI and Diabetes Mellitus (DM). In these two placebo-controlled, double-blind, adaptive Phase III trials, patients with CLI and DM with severe ischemic limb pain will be treated with REX-001 or a placebo to assess the efficacy and safety of REX-001 in the relief of CLI-associated resting limb pain. In a second leg to this Phase III study, CLI patients with ischemic limb pain and skin ulcers (Rutherford stage 5) will be treated with REX-001 or a placebo to ascertain the efficacy and safety of REX-001 in the healing of CLI-induced skin ulcers. The relief of pain and the complete healing of ulcers are the primary endpoints of this clinical trial and amputation-free survival is the secondary endpoint in both legs of the study.
The European Medicines Agency (EMA) has fully endorsed the design of both legs of the trial, and this includes the target patient population and primary endpoints. Clinical improvement in CLI patients increases the probability of successful treatment. Rexgenero also plans to enroll a total of 138 patients at approximately 35 clinical sites in Europe with first interim results expected in circa 18 months’ time and full data in 2020.
Joe Dupere, Rexgenero’s CEO, said “We are extremely pleased to announce the first patient infusion in our Phase III program with REX-001, which if successful could significantly improve the treatment of patients with CLI.” He continued: “The program has been designed following advice from the EMA and in close collaboration with our Scientific Advisory Board. CLI is a medical condition with a clear need for new improved treatment options. Our goal is to bring innovative cell, gene and tissue therapies to the market addressing high unmet needs which cannot be treated with available therapies. We believe that REX-001 has the potential to be one of the first effective cell therapy products available for patients with CLI.”
This is definitely one clinical trial to keep an eye on. If bone-marrow-based cell preparations can help patients sprout new blood vessel networks, there might be applications for other medical conditions as well, including cardiac ischemia, intestinal blood vessel blockage, and liver disease too.
