By Jocelyn Solis-Moreira Aug 18 2021
All COVID-19 vaccines appear less effective when faced with variants such as Delta, according to a new medRxiv* study. However, the researchers suggest that booster shots may help improve immunity and protect against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
The study authors write:
“Modelling of predicted vaccine efficacy against variants over time suggests that protection against symptomatic infection may drop below 50% within the first year after vaccination for some current vaccines. Boosting of previously infected individuals with existing vaccines (which target ancestral virus) has been shown to significantly increase neutralizing antibodies.”
The current study results support recent real-world data from Israel that COVID-19 vaccine immunity wanes after 8 months. As a result, president Biden of the United States recently announced plans to offer booster shots starting in early September for people who received the two-dose mRNA vaccines.
Study: SARS-CoV-2 variants: levels of neutralisation required for protective immunity. Image Credit: NIAID
Variants of concern
Data for modeling were gathered from previous studies exploring neutralized SARS-CoV-2 variants in vitro and in clinical settings.
The researchers first looked at how much neutralizing activity is lost when faced with a variant of concern such as Alpha, Beta, Gamma, and Delta. They analyzed previous data from 16 other studies comparing vaccine effectiveness against the original coronavirus strain from Wuhan and the variants of concern.
While there was some variation in results from different protocols and assays used, further analysis showed that all COVID-19 vaccines were always less effective against variants regardless of type.
A vaccine’s neutralizing activity significantly correlated with the amount of protection against COVID-19.
Severe SARS-CoV-2 infection
The researchers acknowledge that it was hard to predict vaccine efficacy against severe COVID-19 illness because only a few cases were reported.
The results suggest that vaccination continues to protect well against severe COVID-19 illness rather than symptomatic infection.
“It should be noted that our model assumes neutralization alone drives protection against severe disease, but it is likely that other cellular responses play a critical role in modulating disease severity, and thus the model may underestimate efficacy against severe COVID-19,” the researchers explained.
Previous infection
Given waning immunity and recent data on breakthrough infections, booster shots may strengthen the immune system and provide more protection against variants.
Previous studies examining neutralizing antibodies after vaccinations have suggested that one dose of an mRNA vaccine is enough to augment the immune response in people who recovered from SARS-CoV-2 infection.
Clinical trial data evaluating neutralization titers via convalescent plasma showed that an additional dose of the mRNA vaccine in individuals who previously had a COVID-19 infection boosted neutralization levels by 12-fold. The additional dose also improved protection across a spectrum of variants.
“Assuming that the decay of neutralisation titres after boosting is consistent with decay after primary infection, vaccination of convalescent individuals is predicted to provide 69% protection from symptomatic infection and 94% protection from severe infection even against the most escaped VOC (beta) 6-months after boosting,” wrote the researchers.
The results suggest a booster shot could help increase cross-reactivity against several variants of concern in previously infected individuals. In addition, the extra protection is predicted to help against symptomatic and severe COVID-19 illness.
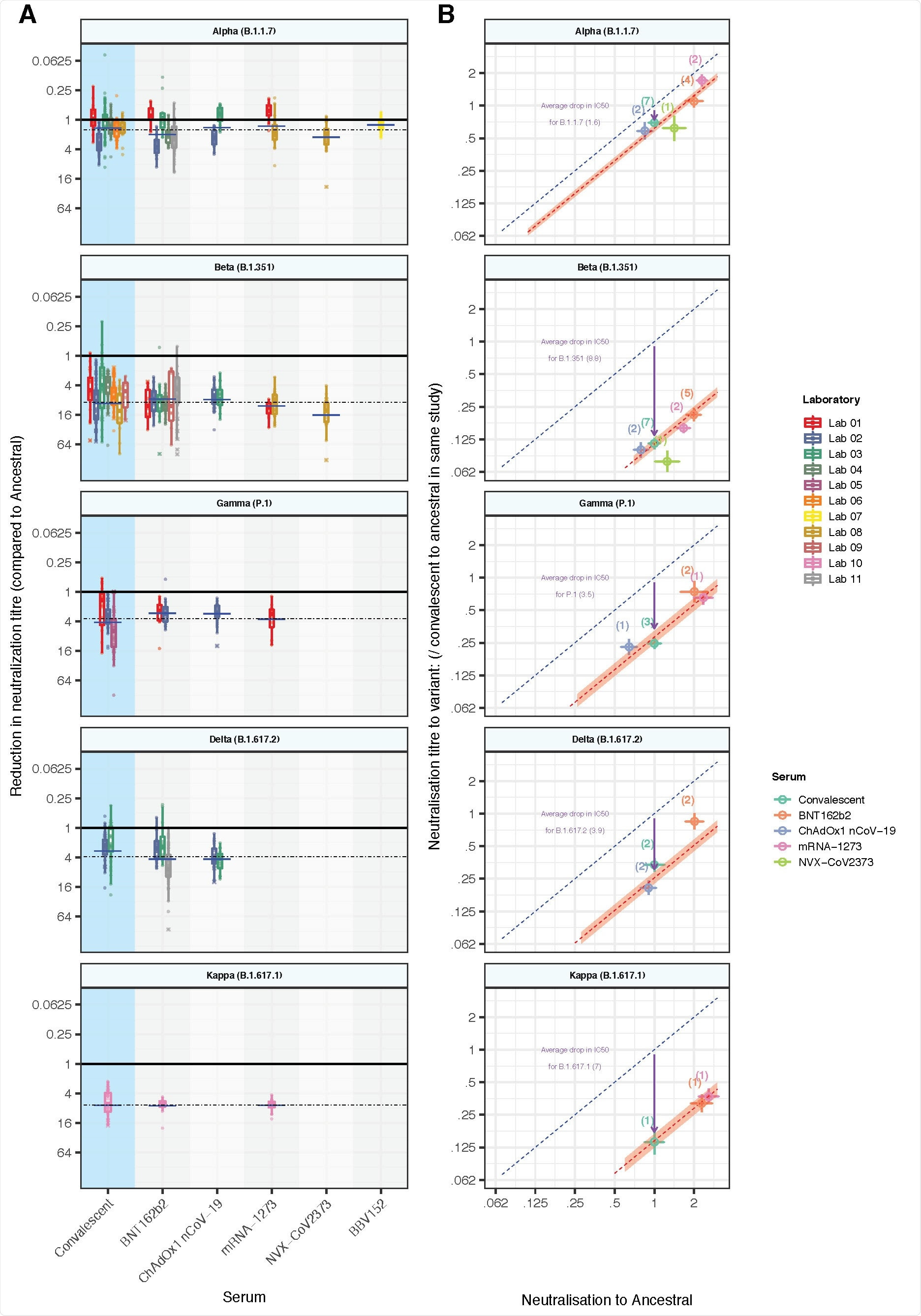
In vitro neutralization of SARS-CoV-2 variants: (a) The change in neutralization titer between the ancestral virus and different SARS-CoV-2 variants for either convalescent individuals (left) or those immunized with different vaccines is shown. Individual colors reflect different studies/laboratories (described in detail in Supplementary Table 1). Solid dots indicate where titers were measurable for both ancestral and variant neutralization. Crosses indicate where one titer fell below the limit of detection for that assay. Different studies estimate quite different changes in neutralization titer even for the same vaccine/variant combination. The dashed horizontal line indicates the weighted mean drop in titre for a given variant (across all vaccine and convalescent samples), and horizontal bars indicate the weighted mean titer for a given vaccine/variant combination. (b) The mean neutralization titer against the ancestral virus (x-axis) is highly correlated with mean neutralization titre against the VOC (y-axis). The predicted line for a 1:1 relationship is indicated (dashed blue line). The observed mean drop in neutralization titre across all vaccines and convalescent subjects is indicated by an arrow, and the predicted levels of variant neutralization are indicated by a dashed red line (shading indicates 95% CI) are shown. The points indicated are the mean neutralization levels for a given vaccine / variant combination, averaging across available studies (number of studies indicated).
Booster shots
Recent work from other studies suggests vaccine-induced immunity wanes after the first eight months of infection.
With weakening immunity and decreased vaccine effectiveness against a variant of concern, current modeling results predict reduced vaccine protection after 1 year.
Limited data on booster shots in vaccinated individuals suggest a third dose of the Moderna vaccine given six months after the 2-dose regimen increased neutralizing activity by 23-fold. Other reports using an additional CoronaVac shot showed 3 to 5-fold higher neutralization levels than neutralization levels with two doses.
The study’s modeling results suggest increases in neutralizing activity — similar to the boost observed in previously infected individuals given the vaccine — is achievable with an additional booster shot for mRNA vaccines.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.

Leave a Reply