by Zen Logsdon, City of Hope National Medical Center
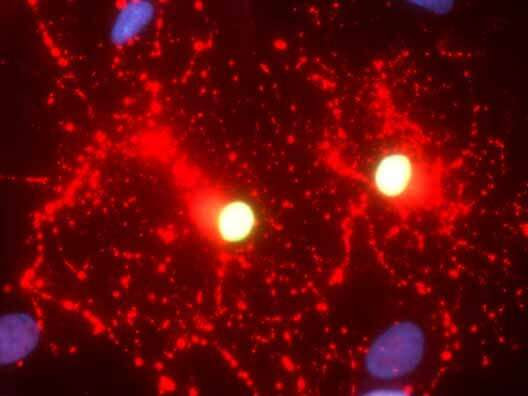
City of Hope’s Yanhong Shi, Ph.D., and her colleagues engineered healthy human skin cells containing the functional aspartoacylase (ASPA) gene into induced pluripotent stem cells (iPSCs) and then differentiated the iPSCs into oligodendroglial progenitor cells, the precursor cells that produce myelin, an insulating sheath that wraps around nerve fibers. Like a bullet train, myelin sheaths facilitate the light-speed transport of information along neuronal axons. Credit: City of Hope
Scientists at City of Hope have developed universal donor stem cells that could one day provide lifesaving therapy to children with lethal brain conditions, such as Canavan disease, as well as to people with other degenerative diseases, such as Alzheimer’s and multiple sclerosis. The study was recently published in Advanced Science.
“The off-the-shelf approach City of Hope is taking can easily be extended to improve the quality of life of cancer patients who are experiencing cognitive impairment or impaired motor function as a side effect of chemotherapy or radiation,” said Yanhong Shi, Ph.D., chair of the Department of Neurodegenerative Diseases and the Herbert Horvitz Professor in Neuroscience at Beckman Research Institute of City of Hope. Shi has been working on this research for 12 years.
This is the first time stem cells have been engineered to become universal donors for cell therapy targeting diseases of the central nervous system, Shi said. This “off-the-shelf” approach can provide patients who need cell therapy with lifesaving treatments three to six months earlier.
Shi and her colleagues engineered healthy human skin cells containing the functional aspartoacylase (ASPA) gene into induced pluripotent stem cells (iPSCs) and then differentiated the iPSCs into oligodendroglial progenitor cells, the precursor cells that produce myelin, an insulating sheath that wraps around nerve fibers. Like a bullet train, myelin sheaths facilitate the light-speed transport of information along neuronal axons.
The treated Canavan disease animal models exhibited increased ASPA activity compared to the control mice and had a reduction in the toxic accumulation of the metabolite N-acetyl-L-aspartate (NAA) in the brain. Too much NAA has been linked to impaired motor function, mental deficiencies and premature death. As a result, the treated mice exhibited increased myelination and vastly improved motor function. Importantly, the universal donor cells were able to evade immune attack from the recipient mice.
While the previous technique her team established created a cell therapy from a patient’s own cells to avoid immune rejection, this new approach used techniques that allowed engineered hypoimmunogenic cells from a healthy donor to be transplanted into a humanized disease model without activating the immune system to kill the foreign therapeutic.

Leave a Reply