by Charlene N. Rivera-Bonet, University of Wisconsin-Madison
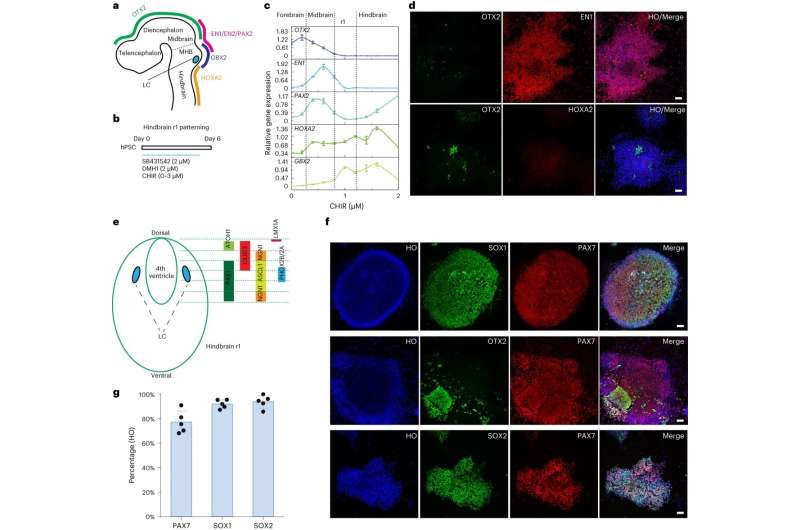
Specification of dorsal hindbrain (r1) neuroepithelia. a, Schematic representation of LC location along the embryonic forebrain, midbrain and hindbrain and their corresponding homeodomain transcription factors. b, Experimental design to pattern hindbrain r1 region from hPSCs during the first 6 d of neural induction. c, Expression of forebrain, midbrain and hindbrain genes under a series of CHIR99021 (CHIR) concentrations. Data are shown as mean ± s.e.m. n = 3 biologically independent samples for each condition. d, Immunostaining for OTX2, EN1 and HOXA2 in day 6 cells when treated with 1.0 µM CHIR99021 in the presence of SB431542 and DMH1. Scale bar, 50 µm. e, Schematic representation of the hindbrain r1 domains along the dorsal to ventral subdomains and their corresponding transcription factors. f, Immunostaining for PAX7, SOX1 and SOX2 at day 6 from cells treated with 1.0 µM CHIR99021 in the presence of SB431542 and DMH1. HO, Hoechst. Scale bar, 50 µm. g, Quantification of SOX2-, SOX1- and PAX7-expressing cells at day 6 when treated with 1.0 µM CHIR99021. Data are shown as mean ± s.d. n = 5 biologically independent samples for each condition. Credit: Nature Biotechnology (2023). DOI: 10.1038/s41587-023-01977-4
Researchers at the University of Wisconsin–Madison have identified a protein key to the development of a type of brain cell believed to play a role in disorders like Alzheimer’s and Parkinson’s diseases and used the discovery to grow the neurons from stem cells for the first time.
The stem-cell-derived norepinephrine neurons of the type found in a part of the human brain called the locus coeruleus may enable research into many psychiatric and neurodegenerative diseases and provide a tool for developing new ways to treat them.
Yunlong Tao, an investigator at Nanjing University in China who was a research professor at UW–Madison’s Waisman Center when the study was performed, and Su-Chun Zhang, a UW–Madison professor of neuroscience and neurology, published their work on the cells, which they call LC-NE neurons, today in the journal Nature Biotechnology.
Norepinephrine neurons in the locus coeruleus regulate heartbeat, blood pressure, arousal, memory, attention and “fight or flight” reactions. Humans have approximately 50,000 LC-NE neurons in the hindbrain, where the locus coeruleus is. From there, the LC-NE neurons reach into all parts of the brain and the spinal cord.
“The norepinephrine neurons in the locus coeruleus are essential for our life. We call it the life center,” Zhang says. “Without these nerve cells, we would probably be extinct from Earth.”
These neurons also play a role, albeit unknown, in various neurodegenerative and neuropsychiatric diseases. In many neurodegenerative diseases such as Alzheimer’s and Parkinson’s, the neurons start degenerating at a very early stage—sometimes years before other brain regions begin to falter.
“People have noticed this for a long time, but they don’t know what the function of the locus coeruleus is in this process. And partly because we don’t have a good model to mimic the human LC-NE neurons,” says Tao, first author of the study.
Previous attempts at creating these neurons from human stem cells followed a protocol based on the development of LC-NE neurons in mouse models. For two years, Tao explored why these attempts were failing and how development of the neurons from stem cells was different in humans.
In the new study, he identified ACTIVIN-A, a protein that belongs to a family of growth factors, as important in regulating neurogenesis in human NE neurons.
“We have some new understanding about locus coeruleus development,” Tao says. “That’s the major finding in this paper, and based on that finding, we are able to generate locus coeruleus norepinephrine neurons.”
To create LC-NE neurons, the researchers converted human pluripotent stem cells into cells from the hindbrain. Then, using ACTIVIN-A and a series of additional signals, they steered cell development toward their fate as LC-NE neurons.
Once converted, the cells showed typical characteristics of functioning LC-NE neurons in the human brain, releasing the neurotransmitter norepinephrine. They also showed axonal arborization—extension of the long, branching arms of neurons that enable the connections between brain cells—and reacted to the presence of carbon dioxide, which is crucial for breathing control.
The new cells may serve as models for disease in humans, allowing scientists to screen drugs for potential treatments and answer questions such as why the cells in the locus coeruleus die so early in neurodegenerative diseases.
“If this is somewhat causative, then we could potentially do something to prevent or delay the neurodegeneration process,” Zhang says.
The LC-NE cells may someday serve as stem-cell therapy themselves.
“The application of these cells is quite broad in its significance,” Zhang says.
Next, the researchers plan to examine the detailed mechanisms through which ACTIVIN-A regulates LC-NE neuron development. The group will also use the cells for the translational work of drug screening and disease modeling.
More information: Yunlong Tao et al, Generation of locus coeruleus norepinephrine neurons from human pluripotent stem cells, Nature Biotechnology (2023). DOI: 10.1038/s41587-023-01977-4
Journal information: Nature Biotechnology
Provided by University of Wisconsin-Madison

Leave a Reply