September 3, 2024
by Maria Fernanda Ziegler, FAPESP
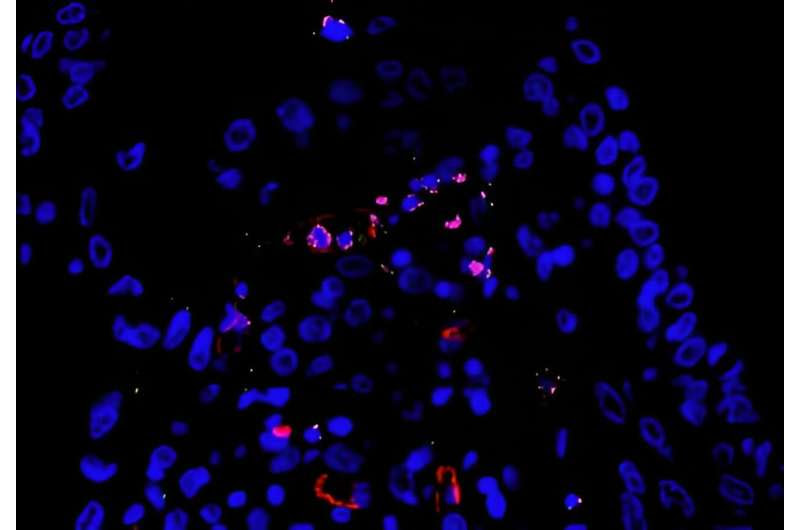
Lung autopsy tissue infected by SARS-CoV-2 with endothelial cells (red), active inflammasome (green) and SARS-CoV-2 RNA (pink), showing that the virus was replicating. Credit: Keyla de Sá
According to an article published in the journal PLOS Pathogens, severe COVID-19 patients can be divided into two distinct groups: those with a high viral load and relatively little inflammation, and those who continue to suffer from inflammatory complications even after the virus has been completely eliminated.
The article describes a study conducted in Brazil by researchers at the University of São Paulo (USP) who analyzed lung autopsies from 47 fatal COVID-19 cases alongside their inflammatory profile, viral load, and immune system activation.
All samples came from patients infected in the first phase of the pandemic, when the ancestral strain of SARS-CoV-2 (first detected in Wuhan, China) was still circulating and no vaccines were available.
Dario Zamboni, full professor at the Ribeirão Preto Medical School (FMRP-USP) and last author of the article, said, “Things have changed since then. There are new variants, and people who have been vaccinated have an infinitely better immune response than people who haven’t.
“Studying these samples [from patients who died after being infected by the ancestral strain in the pre-vaccine phase] is very important to help us understand the molecular mechanisms involved in fatal cases of COVID-19.”
The findings also show why there are so many clinical variations in severe COVID-19 cases and which factors at the molecular level can lead the disease to follow one of the two paths described in the article, as well as supporting decisions on the treatment of critical cases, Zamboni explained.
Exacerbated inflammation
The researchers determined from their analysis of the data that the profile comprising low viral load plus exacerbated inflammation was associated with overactivation of the inflammasome, a protein complex present in the interior of defense cells. When this cellular machinery is activated, pro-inflammatory molecules called cytokines are produced to warn the immune system that more defense cells need to be sent to the site of the infection.
In patients with this profile, inflammasome overactivation caused an exaggerated systemic inflammatory response known as a cytokine storm that injured the lungs and other organs.
“The inflammasome is one of the first responses to infection. Simply put, when macrophages [front-line immune system cells] phagocytize the pathogen, they activate the inflammasome in an attempt to eliminate the infection site. The problem is that several viruses, including SARS-CoV-2, are able to ‘trick’ the immune system in some unknown way and replicate effectively despite inflammasome activation.
“The inflammasome remains active, triggering more inflammation and aggravating the patient’s clinical condition,” said Keyla Sá, first author of the article.
Based on this finding, the researchers conducted experiments with mice genetically engineered to express the protein ACE2, which in humans is the receptor to which the virus binds in order to invade the host’s cells. A key inflammasome gene was “switched off” in some of the mice, so that the cellular machinery responsible for triggering the production of inflammatory cytokines was not activated in response to infection by SARS-CoV-2.
“When we inhibited the inflammasome in infected mice, they became less sick and survived the infection much more. It seems that this ability to elude the immune system may contribute to the significant variation we observed in severe COVID-19 patients. The discovery may make the inflammasome and related factors important targets for novel treatments in future,” Sá said.
Vascular dysfunction
The disease progressed quite differently in fatal cases with high viral load and low inflammation. Evidence of pulmonary thrombosis and disseminated intravascular coagulation led the researchers to conclude that vascular dysfunction culminating in a thrombotic process had a significant impact on the clinical outcome.
“This systematization raises several questions that will be investigated in future. We identified two types of patient who died for different reasons despite having the same disease. The group of patients who eliminated the virus but had exacerbated lung inflammation [including pulmonary fibrosis] died for pulmonary reasons, whereas those with a high viral load had lungs in good condition and were recovering until something else killed them, probably vascular dysfunction,” Sá said.
The two groups also differed sharply in terms of the time between infection and death. The patients with a high viral load died more quickly, while those with exacerbated inflammation spent many days in intensive care and required mechanical ventilation.
“The discovery of these two paths contributes to our understanding of the physiopathology of the disease and may help physicians choose between immune-mediated and antiviral therapies in critical cases of COVID-19,” Zamboni said.
More information: Keyla S. G. de Sá et al, Pulmonary inflammation and viral replication define distinct clinical outcomes in fatal cases of COVID-19, PLOS Pathogens (2024). DOI: 10.1371/journal.ppat.1012222
Journal information: PLoS Pathogens
Provided by FAPESP

Leave a Reply