by Melissa Rohman, Northwestern University
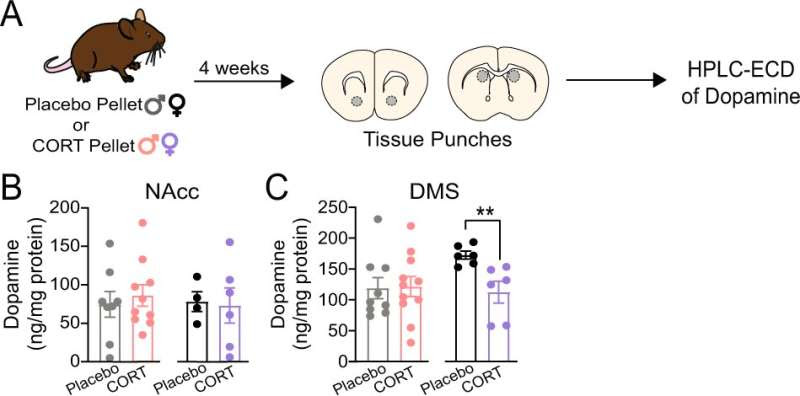
Chronic corticosterone treatment significantly decreases tissue dopamine content of the dorsomedial striatum (DMS) in female mice. A Experimental timeline for pellet implantation, tissue punches, and HPLC-ECD of dopamine. B Tissue dopamine content of the nucleus accumbens core (NAcc) of placebo- and CORT-treated male and female mice. C Tissue dopamine content of the DMS of placebo- and CORT-treated male and female mice. Unpaired two-tailed t-test, **p < 0.01. Each point represents an individual. Data presented as mean ± SEM. Credit: Neuropsychopharmacology (2023). DOI: 10.1038/s41386-023-01551-1
Northwestern Medicine investigators have discovered novel sex-specific mechanisms that control how stress hormones impact dopamine transmission and motivation, findings that can inform new therapeutic strategies for treating major depressive disorder, according to a study published in the journal Neuropsychopharmacology.
“Our discovery of a sex difference in the mechanism by which dopamine transmission is affected by corticosterone treatment adds to a growing body of literature indicating that males and females can display different underlying mechanisms to achieve similar functional or behavioral outcomes. Therefore, it is important not to assume that a lack of observed sex differences at a high level of analysis precludes sex differences in mechanism,” said Talia Lerner, Ph.D., assistant professor of neuroscience and senior author of the study.
An estimated 5% of adults globally are diagnosed with major depressive disorder (MDD) annually and it is a leading cause of disability worldwide, according to the World Health Organization. People diagnosed with MDD exhibit low motivation to engage in rewarding activities, which can decrease overall quality of life.
In a subset of MDD patients, chronic dysregulation of the body’s hypothalamic-pituitary-adrenal (HPA) axis—a neuroendocrine system consisting of the hypothalamus, pituitary glandand adrenal glands—results in increased levels of the stress hormone, cortisol, during the body’s normal resting period.
Stress hormones like cortisol help regulate the body’s adaptive response to change, but chronic stress-induced changes in cortisol can contribute to the development of psychiatric conditions such as MDD.
According to Lerner, there are established sex differences in HPA axis function and regulation. For example, men are more likely to have a dysregulated HPA axis associated with depressive symptoms, while women have higher levels of binding proteins for stress hormones that may prevent HPA axis dysregulation.
In the study, Lerner’s team was interested in understanding how chronic disruptions in stress hormone levels might impair dopamine transmission to produce behavioral symptoms similar to those seen in depression. Using subcutaneous implants, the investigators chronically administered the stress hormone corticosterone to mice; corticosterone is the rodent equivalent of human cortisol.
Using molecular and imaging techniques to study changes in the mouse brains, the team discovered that chronic dysregulation of corticosterone impaired dopamine transmission in the dorsomedial striatum (DMS), a brain area that is essential for associative learning and decisions to seek rewards. Impaired DMS dopamine function was associated with impaired motivation to seek rewards in both sexes. However, the impairments appeared to arise through sex-specific mechanisms.
In male mice, corticosterone dysregulation reduced the function of the dopamine transporter in the DMS. Comparatively, in female mice, corticosterone dysregulation decreased the amount of dopamine in the DMS altogether.
The findings underscore the importance of considering sex, gender and hormone status when designing MDD treatments for humans, according to the authors. In male patients, this newly identified mechanism could help inform therapeutic interventions that target dopamine transporter function to improve motivation. Whereas in female patients, other approaches are likely needed. The use of hormonal birth control methods should also be considered in treatment development, as gonadal hormones interact with HPA axis function, according to Lerner.
“Therapeutic approaches targeting dopamine transporter function could be helpful, but we also need to remember that they might be more effective in men than women when translating these findings to humans. Human data, like animal data, must be carefully analyzed by sex,” Lerner added.
Additionally, screening MDD patients for HPA axis dysregulation could also be an effective therapeutic strategy, according to Ashley Holloway, a student in the Interdepartmental Neuroscience (NUIN) Ph.D. program and lead author of the study.
“Our study emphasizes the need to study the effects of stress hormones in both sexes in order to fully understand their effects on neurobiology and behavior,” Holloway said. “If we screen patients for HPA axis dysregulation, then we may be able to narrow down which therapeutics work best for them based on preclinical research like ours.”
Lerner said her lab is now following up on these initial research efforts, including designing studies to further elucidate the mechanisms by which stress hormones specifically regulate dopamine in men versus women and how normal circadian rhythms in stress hormone release can impact motivation.
“We must continue to probe for sex differences at the molecular level if we are to appropriately translate preclinical discoveries into medicines that act at the molecular level,” Lerner said.

Leave a Reply