Peer-Reviewed Publication
UNIVERSITY OF MONTREAL
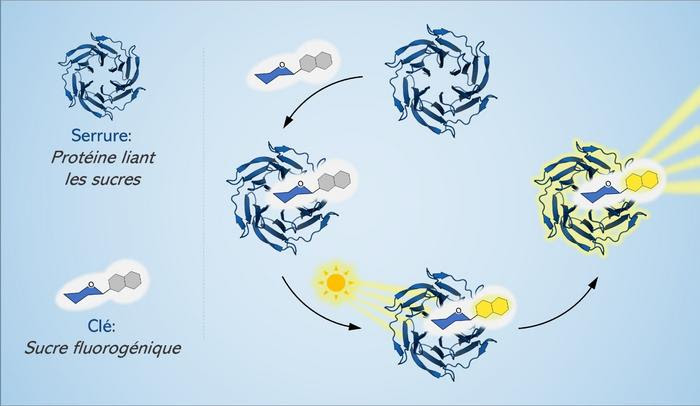
“OUR IDEA WAS TO LABEL SUGAR MOLECULES WITH A CHROMOPHORE, A CHEMICAL THAT GIVES A MOLECULE ITS COLOUR,” EXPLAINED CECIONI. “THE CHROMOPHORE IS ACTUALLY FLUOROGENIC, WHICH MEANS THAT IT CAN BECOME FLUORESCENT IF THE BINDING OF SUGAR WITH THE LECTIN IS EFFICIENTLY CAPTURED.”
CREDIT: CECIONI LAB
Scientists at Université de Montréal’s Department of Chemistry have developed a new fluorogenic probe that can be used to detect and study interactions between two families of biomolecules essential to life: sugars and proteins.
The findings by professor Samy Cecioni and his students, which open the door to a wide range of applications, were published in mid-October in the prestigious European journal Angewandte Chemie.
Found in all living cells
Sugar is omnipresent in our lives, present in almost all the foods we eat. But the importance of these simple carbohydrates extends far beyond tasty desserts. Sugars are vital to virtually all biological processes in living organisms and there is a vast diversity of naturally occurring sugar molecules.
“All of the cells that make up living organisms are covered in a layer of sugar-based molecules known as glycans,” said Cecioni. “Sugars are therefore on the front line of almost all physiological processes and play a fundamental role in maintaining health and preventing disease.”
“For a long time,” he added, “scientists believed that the complex sugars found on the surface of cells were simply decorative. But we now know that these sugars interact with many other types of molecules, in particular with lectins, a large family of proteins.”
Driving disease, from flu to cancer
Like sugars, lectins are found in all living organisms. These proteins have the unique ability to recognize and temporarily attach themselves to sugars. Such interactions occur in many biological processes, such as during the immune response triggered by an infection.
Lectins are attracting a lot of attention these days. This is because scientists have discovered that the phenomenon of lectins “sticking” to sugars plays a key role in the appearance of numerous diseases.
“The more we study the interactions between sugars and lectins, the more we realize how important they are in disease processes,” said Cecioni. “Studies have shown how such interactions are involved in bacteria colonizing our lungs, viruses invading our cells, even cancer cells tricking our immune system into thinking they’re healthy cells.”
Difficult to detect…until now
There are still many missing pieces in the puzzle of how interactions between sugars and lectins unfold because they are so difficult to study. This is because these interactions are transient and weak, making detection a real challenge.
Two of Cecioni’s students, master’s candidate Cécile Bousch and Ph.D. candidate Brandon Vreulz, had the idea of using light to detect these interactions. The three researchers set to work to create a sort of chemical probe capable of “freezing” the meeting between sugar and lectin and making it visible through fluorescence.
The interaction between sugar and lectin can be described using a “lock and key” relationship, where the “key” is the sugar and the “lock” is the lectin. Chemists have already created molecules capable of blocking this lock-and-key interaction, and can now to identify exactly what sugars are binding to lectins of high interest to human health.
“Our idea was to label sugar molecules with a chromophore, a chemical that gives a molecule its colour,” explained Cecioni. “The chromophore is actually fluorogenic, which means that it can become fluorescent if the binding of sugar with the lectin is efficiently captured. Scientists can then study the mechanisms underlying these interactions and the disturbances that can arise.”
Cecioni and his students are confident their technique can be used with other types of molecules. It may even be possible to control the colour of new fluorescently labelled probes that are created.
By making it possible to visualize interactions between molecules, this discovery is giving researchers a valuable new tool for studying biological interactions, many of which are critical to human health.
About this study
“Fluorogenic photo-crosslinking of glycan-binding protein recognition using a fluorinated azido-coumarin fucoside,” by Cécile Bousch et al., was published Oct. 17, 2023 in Angewandte Chemie. Funding was provided by NSERC, the Fonds de recherche du Québec and the Canada Foundation for Innovation.
More on Samy Cecioni
Recruited by UdeM’s Department of Chemistry in 2019, Samy Cecioni is a young researcher specializing in the emerging fields of biological chemistry and glycomics. He is the recipient of a 2021 Medal for Research Excellence from UdeM’s Faculty of Arts and Science.
“Our lab develops new tools designed to accelerate discoveries in the field of glycoscience,” said Cecioni. “Scientists sometimes describe the molecules modified by sugars as the dark matter of biology because they are so difficult to detect. But we are shining a light on these interactions by adopting multidisciplinary approaches at the intersection of chemistry and biology, all with the aim of making significant advances in human health.”
JOURNAL
Angewandte Chemie
DOI
10.1002/anie.202314248
ARTICLE TITLE
Fluorogenic Photo-Crosslinking of Glycan-Binding Protein Recognition Using a Fluorinated Azido-Coumarin Fucoside
ARTICLE PUBLICATION DATE
17-Oct-2023

Leave a Reply