by Justin Jackson, Medical Xpress
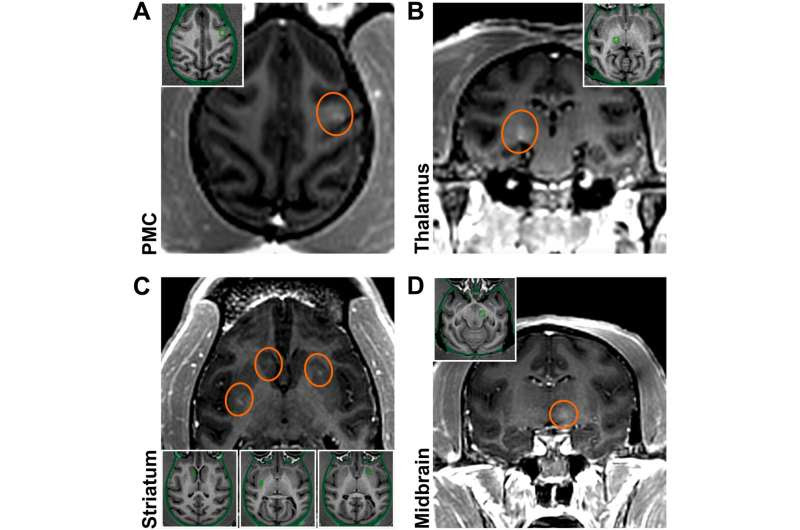
Initial BBB openings in NHPs targeting brain regions relevant to PD. Successful BBB openings were demonstrated in two monkeys (M1/M2) by delivery of an MR contrast agent (Gd) that does not normally extravasate in the brain. Openings were achieved in the targeted regions with relative accuracy. (A) Axial contrast-enhanced T1-weighted MRI image shows BBB disruption in the PMC (indicated by the red circle; M1). (B) Coronal contrast-enhanced MRI shows BBB disruption in the posterior ventrolateral thalamic region (M1). (C) Axial contrast-enhanced MRI shows BBB disruption in the striatum (both the head of the caudate nucleus and the posterior putamen on one side and the anterior putamen on the other; M2). (D) Coronal contrast-enhanced MRI shows BBB disruption in the midbrain (M2). All the enhancements were largely restricted to the targeted regions. Although the same approach was used for each sonication, the size and magnitude of BBB disruption varied depending on the targeted region. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adf4888
In a paper titled “BBB opening with focused ultrasound in nonhuman primates and Parkinson’s disease patients: Targeted AAV vector delivery and PET imaging,” published in Science Advances, researchers led by the University CEU-San Pablo in Spain report on a method of safely traversing the blood-brain barrier.
Neurodegenerative disorders like Parkinson’s, Alzheimer’s and Huntington’s disease have no cure or therapy options to slow their progression. Potentially promising therapy options have been proposed but are limited by the inability to cross the blood-brain barrier.
Gene therapy is one of the potential therapies that researchers would like to study, with the hope of correcting pathogenic mechanisms, bolstering neuroprotection, and initiating neuroregeneration and restoration of damaged brain tissue.
Adeno-associated virus (AAV) vectors are an attractive vehicle for transferring genes into tissues. AAVs are small non-enveloped single-stranded DNA that scientists have been working on to create a platform for gene delivery. There are engineered AAVs with most of the viral genomes replaced with “expression cassettes” containing a promoter, a gene payload, and a terminator. These research AAVs cannot replicate like a wild virus, but the gene cargo they carry can be expressed (often with a second helper virus), providing long-term therapeutically beneficial gene expression.
An advantage of gene therapy is that AAV vector delivery would only occasionally be needed as previous studies have shown treatments that last several years or more. Drug molecule therapies require much more frequent interventions, which is not usually a barrier to treatment unless that treatment is in the brain.
One limiting factor in using AAVs (or drug molecules) in the brain is the blood-brain barrier, a tight net of protective endothelial cells, pericytes, astrocytes and microglia all work to keep out bacteria and unwanted molecules while letting oxygen, carbon dioxide and water circulation occur.
To get past the barrier, AAVs must remain very small, limiting the cassette payload options. Once the AAVs make it past, another problem arises, location. If AAVs are needed to treat specific tissues in specific locations of the brain, just getting past the blood-brain may not be enough to generate the therapeutic effect.
In animal research models, the act of inserting AAVs with intracerebral injections, the most direct way of conducting a therapeutic genetic trial, can sometimes result in enough traumatic tissue damage to cause death. Reasonably, this is a limiting factor when considering human trials. As the study authors point out, neurodegenerative disease treatments may require delivery into several brain regions simultaneously and an ideal gene delivery approach to brain diseases should be safe, noninvasive, and region-specific.
In the current study, researchers report creating a successful blood-brain barrier opening and delivering AAV vectors into specific brain regions involved in Parkinson’s disease using low-intensity focus ultrasound guided by magnetic resonance imaging.
Neuronal green fluorescent protein expression was observed specifically in regions with the blood-brain barrier opening, confirming that the delivery method succeeded. Blood-brain barrier permeability was restored within 24 hours, and no adverse effects were reported.
The initial trial was conducted in adult macaque monkeys, and a follow-up was conducted on three human patients with Parkinson’s disease. The less-invasive nature of this methodology could facilitate viral vector delivery for gene therapy making theoretical interventions to treat neurodegenerative disorders a reality.
While the current study focused on neurodegenerative disorders, a host of other ailments have evaded treatment because they take place behind the blood-brain barrier and are beyond the reach of conventional therapeutics. Meningitis, syphilis and toxoplasmosis, to name a few, could all see new treatment options with a safe passage across the labyrinth of protective brain vasculature

Leave a Reply