SCIENCE & TECHNOLOGY NEWS
Selecting specific cells to be used in an investigational therapy led to improved recovery of heart function in a new study employing a lab model for myocardial infarction, the medical term for heart attacks.
A team led by researchers at the California NanoSystems Institute at UCLA and Columbia University developed a sorting method and grouped stem cells based on how many vesicles involved in cellular communication that they released. Treatment with high-secreting cells restored heart function back to a similar state as before the damage from myocardial infarction.
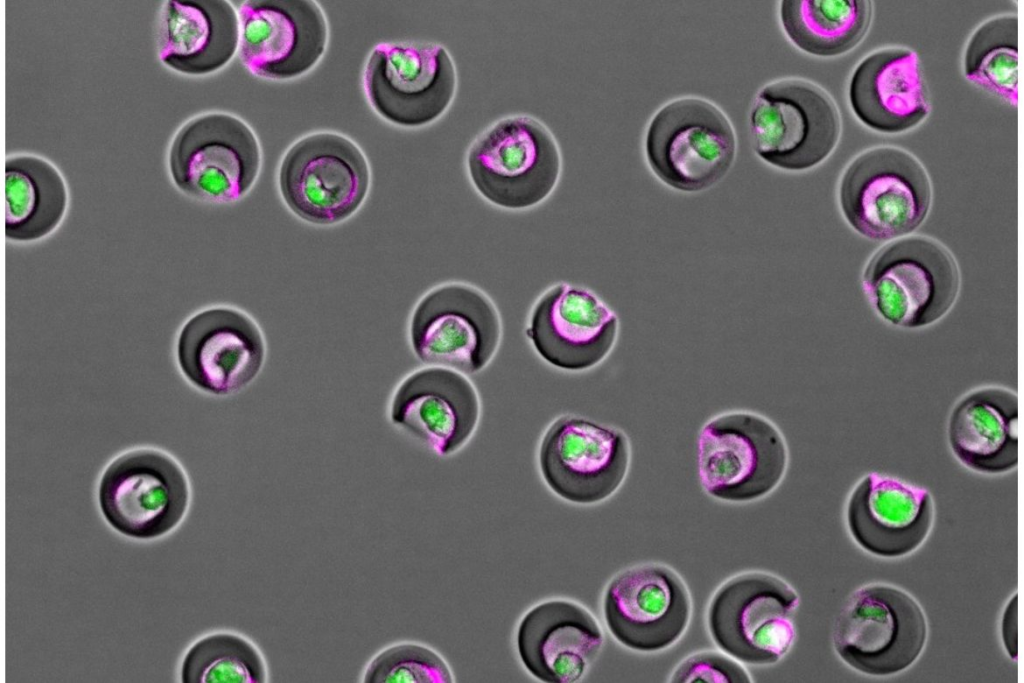
Nanovials containing individual cells (green) and secreted extracellular vesicles (magenta) within their cavities. Image credit: Doyeon Koo/UCLA
BACKGROUND
Numerous treatments under development to fight serious illness use cells as delivery devices for therapeutic molecules or as therapies themselves. Some use mesenchymal stem cells, or MSCs, a population of cells found in the fat tissue or bone marrow. To help against heart disease — the most common cause of death in the U.S. and worldwide — one approach aims to heal damaged heart muscle after myocardial infarction by delivering naturally occurring cell secretions called extracellular vesicles.
Extracellular vesicles are an aspect of how cells in the body communicate with one another. They are made up of waxy, protein-laden membranes that can carry DNA and RNA instructions inside. Often involved in reducing inflammation or promoting healing, these vesicles are hypothesized to deliver substantially more information compared to smaller signaling proteins or hormones.
Although MSC-delivered vesicles have been effective in regenerating heart tissue in preclinical research, that success has been difficult to replicate in studies with patients. Part of the reason may be that the rate of vesicle production can vary widely among MSCs from different patients or tissues. To date, there are no known markers on the cells themselves that can be used to predict which ones will secrete more vesicles, and no methods to sort cells based on this important function.
METHOD
The team sorted MSCs using nanovials, microscopic bowl-shaped hydrogel containers developed by CNSI researchers. The interior of the nanovials were decorated with certain proteins and antibodies that MSCs and vesicles latch onto, enabling the researchers to capture cells individually and filter based on how many vesicles they released using standard instruments in the lab.
When high-secreting MSCs were allowed to grow in culture for two weeks, the expanded populations maintained that high rate of production and grew faster compared to low-secreting MSCs. The scientists evaluated which genes were expressed in the MSCs and found that genes connected to regeneration and blood vessel growth were activated at a greater level in high-secreting cells.
High- and low-secreting MSCs were used to treat myocardial infarction in two groups of mice. After 28 days, both groups showed increases in two measures of heart function, whereas a control group showed a decrease. Notably, those treated with high-secreting cells reached levels approaching the baseline before damage to the heart.
IMPACT
With the study’s evidence that isolating and growing MSCs that release more vesicles can lead to better outcomes after a heart attack, this research could provide a route for realizing the cell therapy’s potential to benefit human health. More broadly, the findings suggest that other cell-based treatments could be more effective if nanovials are used to select for higher production of therapeutic secretions. Sorting for high-secreting cells may likewise make the manufacturing of medicines derived from cells in a lab more efficient.
AUTHORS
The study’s corresponding authors are Dino Di Carlo, the Armond and Elena Hairapetian Professor of Engineering and Medicine in the UCLA Samueli School of Engineering and a member of CNSI and the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, and Ke Cheng, a professor of biomedical engineering at Columbia University. The study’s co-first authors are Doyeon Koo, who earned a doctoral degree from UCLA in 2023, and Xiao Cheng of University of North Carolina at Chapel Hill and North Carolina State University. Other co-authors are Shreya Udani, Sevana Baghdasarian and Natalie Tsubamoto of UCLA; Dashuai Zhu and Shiqi Hu of Columbia; Junlang Li of Xsome Biotech; Brian Hall of Cytek Biosciences; and Jina Ko of the University of Pennsylvania.
DISCLOSURES
Di Carlo and the University of California have financial interests in Partillion Bioscience, which is commercializing nanovial technology.
JOURNAL
The study was published in Nature Communications.
FUNDING
The study was supported by the National Institutes of Health, the American Heart Association and the Chan Zuckerberg Initiative.
Written by Wayne Lewis
Source: University of California

Leave a Reply