by Adrienne Williamson, Medical Xpress
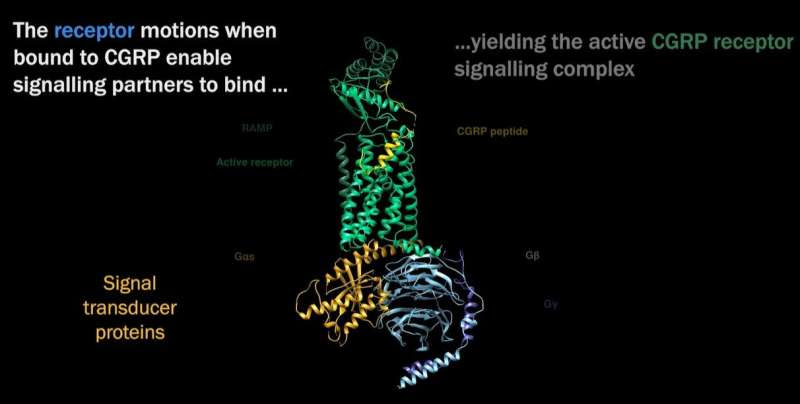
CGRP receptor signaling complex structure. Visualization provided by Patrick M. Sexton, PhD.
A research team with members from Monash University, the ARC Centre for Cryo-electron Microscopy of Membrane Proteins, the University of Tokyo and the University of Otago have determined the shape and kinetics of an important cell surface (membrane) receptor called calcitonin gene-related peptide (CGRP), which has long been implicated in migraine. The work has been published in the journal Science.
Migraine is more than a simple headache; more than 3 million migraineurs—more than 60% of whom are women—have at least one attack per year. A smaller subset experience chronic migraine, defined by migraine pain that occurs 15 days or more per month, for three or months in a row. While sufferers experience many different symptoms of varying intensity—usually nausea, dizziness, sensitivity to light and sound, and intense pain on one or both sides of the head—the physiological process of migraine onset and pain is different from other types of headache, such as muscle tension or sinus pain. While migraine was long believed to be a neurovascular disorder that involved dilation of vessels in the skull, face and cerebral membrane, research has excluded vasodilation as a factor in this type of pain. More recent work has identified increased CGRP in the trigeminal sensory nervous pain pathway that results in headache.
“We determined the atomic structure of an important cell surface (membrane) receptor that is implicated in migraine,” says Dr. Radostin Danev of the Graduate School of Medicine, University of Tokyo. “In this initial study, we determined the structure of the receptor alone and in combination with its natural target molecule (CGRP). This gives us a clear understanding of how the receptor works during its normal function in the body.”
Future studies will expand the investigations towards potential drug targets. “There are already several migraine drugs that target the receptor, but structural knowledge is essential for understanding the pharmacology and for future therapy developments” that will “help us understand the pathology mechanisms of chronic diseases and to aid in the development of more effective and accessible treatments,” says Dr. Danev.
Identifying the structure of CGRP “is one of the first molecular revelations of the initiating events that are associated with migraine pain,” explains Dr. Patrick M. Sexton, Professor of Pharmacology at Monash University and a corresponding author of the paper. “The approach we used to do this (cryo-EM) is similar to the approach used to support vaccine and drug development for COVID.”

The trigeminal nerve is the fifth cranial nerve and is responsible for transmitting sensations from the face, brain lining (meninges), eyes, sinuses, TMJs, ears, salivary glands, mouth, nasal cavity and teeth to the brain. It is composed of three branches; the ophthalmic, maxillary, and mandibular. It is also the nerve that controls the muscles used for chewing. Credit: orofacialpain.org.uk
While this research and all research into COVID-19 is recent, cryogenic electron microscopy (cryo-EM) has been in use since the 1970s. Advances in the past decade have improved detector technology and computational imaging, allowing cryo-EM to reveal biomolecular structures at near-atomic resolution. Dr. Danev is excited for the future of cryo-EM research: “This is the first successful high-resolution cryo-EM study of an isolated G protein-coupled receptor in inactive and pre-activation states, without the presence of a G protein. It showcases the ever-expanding capabilities of cryo-EM for the structure determination of small membrane proteins.” And while the team are thrilled with the results of their work, the real benefit is forthcoming, Dr. Danev says. “This work paves the way towards further studies of inactive GPCRs, which until now have been a prerogative of X-ray crystallography.”
So where do we go now that we have this understanding of CGRP’s structure and the ability to investigate it and others? “The new structures and understanding of the motions of the receptors could be used to design better ways of activating this receptor,” explains Dr. Sexton. “While the work unravels a key event in migraine, we already have treatments recently approved that effectively target the receptor we studied. However, sometimes we will actually want to increase activation of the receptor to treat other diseases.”

Leave a Reply