by Sichuan International Medical Exchange and Promotion Association
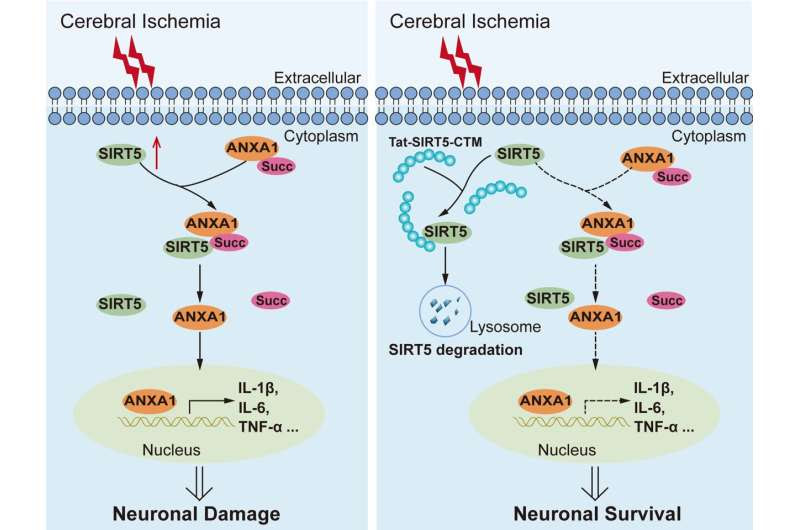
The Tat-SIRT5-CTM peptide specifically blocked the interaction of SIRT5 with ANXA1, led to SIRT5 degradation and thereby inhibiting SIRT5-mediated desuccinylation of ANXA1, which in turn alleviated microglia-induced neuroinflammation, and eventually improved the survival of neurons subjected to cerebral ischemia and reperfusion injury. Credit: Figure was drawn using Adobe illustrator CS6 software.
Stroke is a major public health concern worldwide. The lack of effective therapies heightens the need for new therapeutic agents.
Previous research found that the increase in SIRT5 in microglia induced by ischemic stroke causes annexin-A1 (ANXA1) desuccinylation, which decreases ANXA1 membrane recruitment and secretion but promotes ANXA1 nuclear translocation, results in the production of proinflammatory mediators and ultimately enhancing neuroinflammation damage.
Thus, microglia-specific forced overexpression of SIRT5 worsened ischemic brain injury, whereas downregulation of SIRT5 exhibited neuroprotective and cognitive-preserving effects against ischemic brain injury, as was shown by a previous study published in Journal of Neuroinflammation.
Based on this, a membrane-permeable peptide specifically bound to SIRT5 through a chaperone-mediated autophagy targeting motif (Tat-SIRT5-CTM) was designed and synthesized.
In primary microglia, Tat-SIRT5-CTM suppressed the binding of SIRT5 with ANXA1, leading to SIRT5 degradation and thus inhibition of SIRT5-mediated desuccinylation of ANXA1, followed by increased membrane accumulation and secretion of ANXA1. These changes, in turn, alleviated microglia-induced neuroinflammation.
The team led by Dr. Xing Li and Dr. Yilin Zhao (Department of Anesthesiology, Hubei Key Laboratory of Geriatric Anesthesia and Perioperative Brain Health, and Wuhan Clinical Research Center for Geriatric Anesthesia, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology) sought to determine the therapeutic effect of this cell-penetrating peptide in mice subjected to ischemic stroke. Following intravenous injection, Tat-SIRT5-CTM could efficiently pass through the blood‒brain barrier.
The study, “A cell-penetrating peptide exerts therapeutic effects against ischemic stroke by mediating the lysosomal degradation of sirtuin 5,” is published in the journal MedComm.
Importantly, systemic administration of Tat-SIRT5-CTM reduced the brain infarct area and neuronal loss, mitigated neurological deficit scores, and improved long-term neurological functions in a mouse model of ischemic stroke. Furthermore, no toxicity was observed when high doses Tat-SIRT5-CTM were injected into nonischemic mice.
The team further indicated that the Tat-SIRT5-CTM inhibits the expression of inflammatory cytokines in microglia after OGD/R or in a mouse model of ischemic stroke. “TUNEL staining showed that microglia or mice treated with Tat-SIRT5-CTM can protect against neuronal damage under OGD/R or MACO/R conditions,” first author Qian Xia says.
The team also found that the Tat-SIRT5-CTM reduces the infarct volume and mitigates the neurological deficit scores (see left and center images below). “The TTC staining confirmed Tat-SIRT5-CTM successfully reduced the cerebral infarct size, and the modified neurological severity scores demonstrated that Tat-SIRT5-CTM displayed a better neurological score,” Qian Xia says.
A novel cell-penetrating peptide exerts therapeutic effects against ischemic strokeOn the left are the representative images of TTC-stained tissues from mice treated with the Tat-SIRT5-CTM peptide. In the center is the mNSS results showing the extent of neurological deficits at 72 h after reperfusion. On the right is the time of the mice falling off the rotarod drum during the final rotarod test. Credit: Qian Xia
The researchers finally assess long-term neurobehavioral functions after ischemic brain injury with a battery of behavioral tests. In the rotarod test, the results showed that Tat-SIRT5-CTM-treated mice took longer to fall off the rod and preserves long-term neurobehavioral functions after ischemic brain injury (see below, right image).
“These new exciting results add to growing evidence that promising efficacy of the peptide-directed lysosomal degradation of SIRT5 and suggests it as an effective therapeutic approach for the treatment of ischemic stroke. It was recently proven through a Phase III clinical trial that a Tat-fused peptide called Nerinetide (NA-1) is not only safe but also effective in preventing ischemic damage to human neurons. We hope that this Tat-SIRT5-CTM peptide can also be successfully translated to the clinic as a new and effective treatment for ischemic stroke in human patients,” Qian Xia says.
More information: Qian Xia et al, A cell‐penetrating peptide exerts therapeutic effects against ischemic stroke by mediating the lysosomal degradation of sirtuin 5, MedComm (2023). DOI: 10.1002/mco2.436
Journal information: Journal of Neuroinflammation
Provided by Sichuan International Medical Exchange and Promotion Association

Leave a Reply