by Melissa Rohman and Olivia Dimmer, Northwestern University
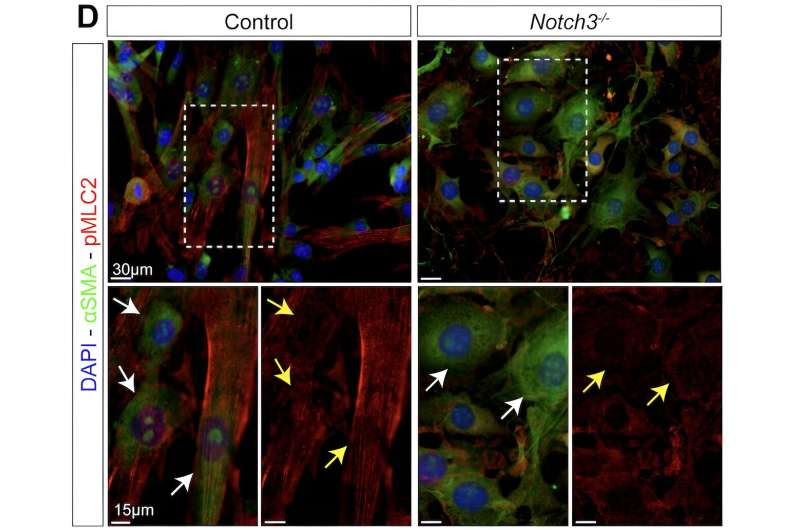
Notch3 deficiency results in vascular dysfunction due to impaired contractility. Immunofluorescence of phosphorylated myosin light chain 2 (pMLC2–red). White arrows highlight alpha-SMA (green), yellow arrows highlight pMLC (red). Credit: Luisa Iruela-Arispe, Ph.D./Northwestern University
Decreased activity of a specific signaling pathway in brain vessels was linked to a decline in vascular function and subsequent neurodegeneration, according to a recent Northwestern Medicine study published in the Journal of Clinical Investigation.
The study is the first to demonstrate that decreased activity of the Notch3 signaling pathway can be used as a biomarker for vascular aging and neurodegeneration, findings that could help inform the development of targeted therapies to prevent and treat age-related neurodegenerative diseases, according to Luisa Iruela-Arispe, Ph.D., the chair and Stephen Walter Ranson Professor of Cell and Developmental Biology, and senior author of the study.
“Our initial goal was to capture changes in the brain vasculature associated with aging. The study is foundational because it provides information related to physiological aging in the absence of any other confounding pathologies,” said Arispe, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
An outcome of normal physiological aging is the progressive decline in the functioning of different organ systems, including the vascular system, which subsequently reduces blood flow to the brain, leading to vascular dementia and neurodegeneration.
Patients with vascular dementia experience cognitive decline, memory loss and changes in behavior. However, the underlying mechanisms of vascular dementia that cause this cascade of symptoms are not well understood.
“The intricate co-dependency between brain function and the vasculature has been well acknowledged by clinicians and scientists, but how the aging vasculature affects the brain is less understood,” Arispe said.
Using single-cell transcriptomics to study brain vessels from young and aging humans and mice, the scientists found in the aging mice a noticeable decline in the activation of the Notch3 signaling pathway. Notch3 signaling makes up one of four Notch receptors, which are essential for cell development and response to cellular stressors.
Credit: Northwestern University
Additional transcriptomics analysis and MRI scans of both mouse and human blood vessels revealed that reduction in Notch3 signaling altered the regulation of calcium and prevented blood vessels from properly contracting, which contributed to multiple vascular impairments, including dilation, microaneurysms, and decreased blood flow to the brain.
Furthermore, the investigators found these vascular impairments prevented proper glymphatic flow, or the clearing of waste and excess extracellular fluid from the central nervous system.
According to the authors, these pathological features are also observed in patients with CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy)—a rare, inherited type of vascular disease that causes dementia and is also associated with Notch3 mutations.
“The fact that we identified Notch3 as the top pathway altered by aging was powerful because of its known association with hereditary vascular dementia,” Arispe said.
According to Arispe, her team’s findings are essential for improving the understanding of what causes normal physiological aging without additional cofounding factors and confirms Notch3 as a biomarker of vascular aging.
Arispe said her team and collaborators are now particularly interested in determining whether intervening to increase Notch3 signaling in small muscle cells located in the wall of blood vessels might rejuvenate contractility, and they also hope to develop more appropriate models of CADASIL to more accurately compare them to the progressive loss of Notch3 function in aging.
“My research group is extremely appreciative of the community of clinicians and scientists here are Feinberg and our extensive group of collaborators that contributed so much to this study and facilitated the extension of these findings to patients,” Arispe said.
More information: Milagros C. Romay et al, Age-related loss of Notch3 underlies brain vascular contractility deficiencies, glymphatic dysfunction, and neurodegeneration in mice, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI166134
Journal information: Journal of Clinical Investigation
Provided by Northwestern University

Leave a Reply