by Laura Muntean, Texas A&M University
Credit: Molecular Metabolism (2023). DOI: 10.1016/j.molmet.2023.101852
A team comprised primarily of Texas A&M AgriLife Research scientists has made an important discovery that could lead to a novel treatment for obesity and obesity-associated diseases or conditions.
Details of the discovery can be found in the study “Nutrient-sensing growth hormone secretagogue receptor in macrophage programming and meta-inflammation,” published in the journal Molecular Metabolism.
“Chronic inflammation commonly associated with obesity is a key reason obese individuals often have many other chronic diseases,” said Yuxiang Sun, who served as lead investigator for the study.
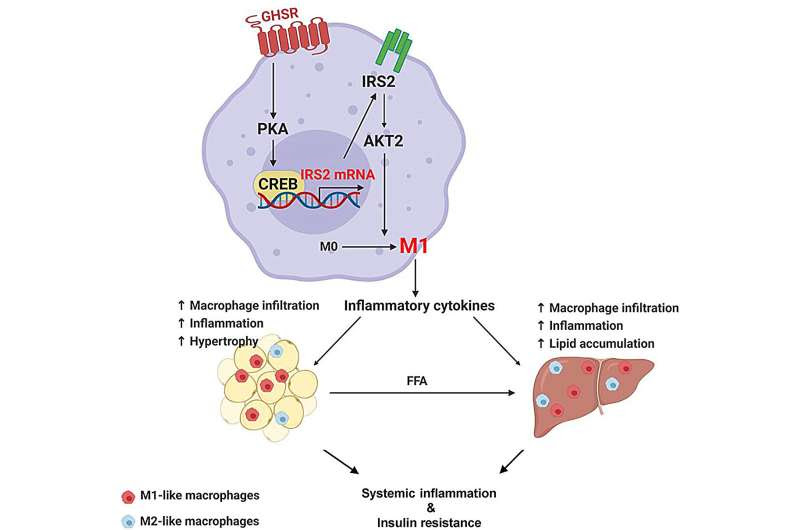
The study focused on the role of one molecule involved in how our bodies deal with hunger: the growth hormone secretagogue receptor, GHSR, which mediates the effect of ghrelin, known as the “hunger hormone.” Studies have shown that ghrelin promotes eating and increases fat. Ghrelin activates GHSR to increase appetite, fat accumulation and insulin resistance.
Research has shown that under normal conditions, GHSR is highly active in the brain but much less active in other tissues such as liver and fat, also referred to as adipose tissue. For this reason, most ghrelin research has focused on the brain.
“Intriguingly, in previous research, we found the complete removal of GHSR protects against diet-induced inflammation and insulin resistance in adipose tissue and the liver without affecting food intake,” Sun said. “This was very puzzling because that GHSR has very low expression in fat and liver cells.”
A link between obesity and the immune system
Sun and her team has made the novel observation that GHSR activity in macrophages—a major immune cell type in tissues—increases dramatically under the condition of obesity. In this study, Sun’s team investigated whether ghrelin’s effect in adipose tissues and the liver was due to the infiltration of GHSR-expressing macrophages into these tissues under the condition of obesity.
“If that was the case, this infiltration by those particular GHSR-expressing macrophages would trigger chronic inflammation and insulin resistance,” Sun said.
Study results and implications
To understand the role of GHSR in macrophages, the team developed a unique animal model to selectively shut down GHSR activity in macrophages.
“Indeed, our study results showed that macrophage-specific GHSR deficiency reduces diet-induced systemic inflammation and insulin resistance,” Sun said. “Remarkably, GHSR deficiency in macrophages reduced diet-induced macrophage infiltration, macrophage activation and fat deposition in adipose tissue and the liver.”
In addition to their effect on diet-induced chronic inflammation, the study also demonstrated that GHSR-deficient macrophages protect against acute inflammation induced by bacterial toxins.
At a molecular level, they found GHSR programs macrophages through an insulin signaling pathway, Sun said. Basically, this study showed that macrophage GHSR controls chronic inflammation in obesity by regulating macrophage programming.
She said the study’s novel results demonstrate that macrophage GHSR plays a key role in meta-inflammation by promoting macrophage infiltration and inflammatory activation.
“These exciting new findings helped to resolve a long-time mystery of GHSR in adipose tissue and the liver in obesity, by uncovering the novel immunoregulatory role of GHSR and revealing that GHSR signaling is a critical link between metabolism and immunity,” Sun said.
She said the study adds a new dimension to the biology of ghrelin, and underscores that ghrelin is not only a hunger hormone, but also an important nutrient-sensor and immune regulator.
“This has profound implications for health and disease, as blocking GHSR in macrophages may serve as a promising immune therapy to prevent or treat obesity, diabetes and inflammation,” Sun said.
More information: Da Mi Kim et al, Nutrient-sensing growth hormone secretagogue receptor in macrophage programming and meta-inflammation, Molecular Metabolism (2023). DOI: 10.1016/j.molmet.2023.101852
Journal information: Molecular Metabolism
Provided by Texas A&M University

Leave a Reply