Cell & Microbiology
Home
Biology
Molecular & Computational biology
JUNE 24, 2024
by Jim Schnabel, Cornell University
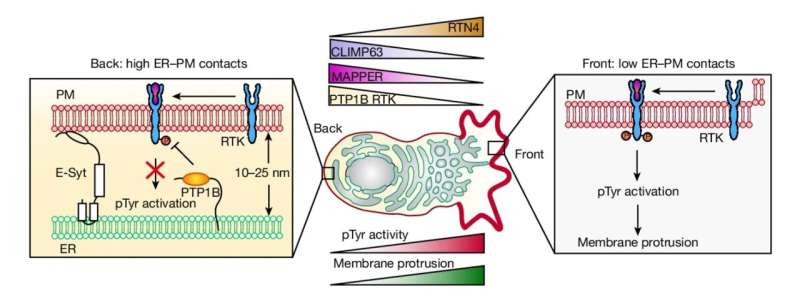
Model of how ER–PM contact gradients generate the observed gradient of pTyr signaling. Credit: Nature (2024). DOI: 10.1038/s41586-024-07527-5
Interactions between two key structures within cells help establish the front-to-back “polarity” that is essential to cell migration, according to a new study by Weill Cornell Medicine researchers.
These migrations occur in organ development, wound healing, cancer metastasis and many other processes, but how moving cells respond to environmental cues and set up internal structures that enable them to keep going in one direction has not been fully clear.
As the researchers report June 12 in Nature, contacts between a prominent inner structure in cells, the endoplasmic reticulum (ER), and the inside of the cells’ plasma membrane (PM) are larger and denser at the back of a migrating cell and smaller and sparser in front. This difference, or “gradient,” appears to be the basic structural determinant of cell motion: When the researchers artificially induced ER-PM contacts at the front of moving cells, the cells reversed direction.
“We think this discovery marks a big shift in understanding cell polarity in the context of cell migration—and likely in some other contexts,” said senior author Tobias Meyer, the Joseph Hinsey Professor of Cell & Developmental Biology at Weill Cornell Medicine.
The study’s first author is Bo Gong, a postdoctoral researcher in the Meyer Laboratory.
Biologists have known for more than two decades that migrating cells use molecules called phospholipids and signaling enzymes called GTPases to establish a front end—from which they extend foot-like protrusions that pull them along surfaces. Yet there have been hints of other mechanisms, including mechanisms that define a migrating cell’s back end.
Gong was looking for a sustained structural basis for this back-end definition when he decided to examine ER-PM contacts. These had important established roles in cell signaling and in regulating properties of the cell membrane but were not known to be spatially organized to regulate cell function. Yet in early experiments, Gong found that these contacts were significantly larger, denser and more stable at the backs of migrating cells compared with the fronts.
“This back-to-front gradient was evident across all the cell types I tested, in flat and 3D environments, and in cells migrating individually or collectively,” Gong said.
When he artificially created ER-PM contacts—like those normally seen at the back end—at the front end of migrating cells, the cells reversed their front-back polarity and their directions of movement. He also observed that the speed of migrating cells was faster when the back-to-front difference in ER-PM contact density was greater.
Underlying this speed-control effect, he found, is an ER-associated phosphatase enzyme that works at a moving cell’s back end—where the better ER-PM contacts make it more available at the membrane—to suppress front-end-type signaling.
The researchers also determined that ER-PM contact density is greater at the back end because the ER has a relatively flat structure there, whereas it is relatively tubular and wavy at the front, and thus can’t contact the membrane as snugly.
Meyer and Gong suspect that, in general, this newly discovered structural polarity of ER-PM contacts in migrating cells is the principal foundation for the signaling polarity and other forms of polarity that have been observed in these cells.
The research, in addition to its value for basic cell biology, could inform future approaches for treating or preventing problematic cell migration such as tumor metastasis. It may also relate to other forms of cell polarity, such as the structural and signaling polarity of neurons, in which mutations of the ER-shaping proteins are associated with neurodegenerative diseases.
“We suspect that this process we have uncovered will be shown to have very broad relevance,” Meyer said.
More information: Bo Gong et al, Endoplasmic reticulum–plasma membrane contact gradients direct cell migration, Nature (2024). DOI: 10.1038/s41586-024-07527-5
Journal information: Nature

Leave a Reply