by University of Arizona
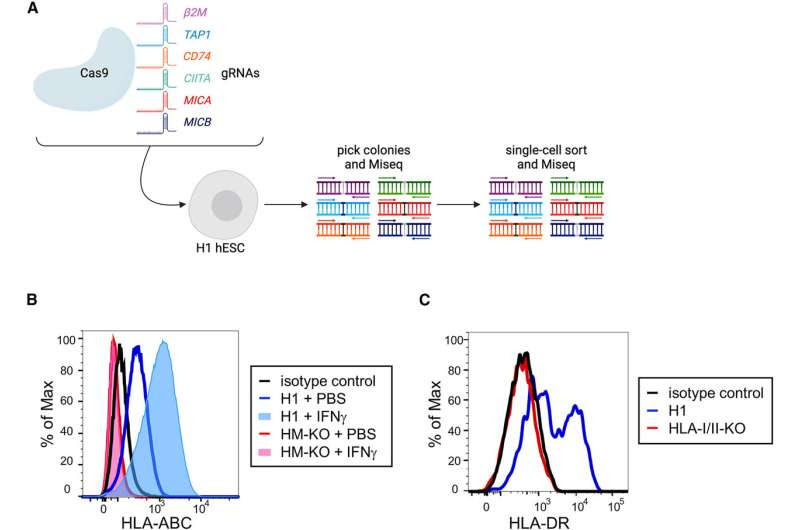
Generation of an HLA-I/II and MICA/B-deficient hESC line (A) Representation of the Cas9 editing strategy. Wild-type H1 hESCs were transfected with a Cas9 construct and up to three gRNAs at a time. gRNAs were for β2M, TAP1, CD74, CIITA, MICA, and MICB. Individual colonies were picked and sequenced to identify ones with frameshift mutations in target genes. Colonies with mutations were clonally sorted and re-sequenced. The resulting cells were then iteratively targeted by gRNAs to generate a line in which all alleles carried frameshift mutations. (B) Representative flow cytometric histograms of HLA-ABC (HLA-I) expression on H1 and HM-KO hESCs treated with PBS or IFNγ. (C) Representative histograms of HLA-DR (HLA-II) expression of DC-like cultures differentiated from H1 or HLA-I/II-KO hESCs. Credit: Stem Cell Reports (2024). DOI: 10.1016/j.stemcr.2023.12.003
One of the biggest barriers to regenerative medicine is immunological rejection by the recipient, a problem researchers at the University of Arizona Health Sciences are one step closer to solving after genetically modifying pluripotent stem cells to evade immune recognition.
Their study titled “Engineering Human Pluripotent Stem Cell Lines to Evade Xenogeneic Transplantation Barriers” is published in the journal Stem Cell Reports.
Pluripotent stem cells can turn into any type of cell in the body. The findings offer a viable path forward for pluripotent stem cell-based therapies to restore tissues that are lost in diseases such as Type 1 diabetes or macular degeneration.
“There has been a lot of excitement for decades around the field of pluripotent stem cells and regenerative medicine,” said principal investigator Deepta Bhattacharya, Ph.D., a professor in the UArizona College of Medicine—Tucson’s Department of Immunobiology.
“What we have learned from the experiences of organ transplantation is that you have to have matched donors, but the person receiving the transplant often still requires lifelong immune suppression, and that means there is increased susceptibility to infections and cancer. We’ve been trying to figure out what it is that you need to do to those stem cells to keep them from getting rejected, and it looks like we have a possible solution.”
To test their hypothesis, Bhattacharya and the research team used CRISPR-Cas9 technology, “genetic scissors” that allow scientists to make precise mutations within the genome at extremely specific locations.
Using human pluripotent stem cells, the team located the specific genes they believed were involved in immune rejection and removed them. Prior research into pluripotent stem cells and immune rejection had looked at different parts of the immune system in isolation. Bhattacharya and his colleagues from The New York Stem Cell Foundation Research Institute, St. Jude Children’s Research Hospital and the Washington University School of Medicine opted to test their genetically modified stem cells in a complete and functional immune system.
“The immune system is really complicated and there are all sorts of ways it can recognize and reject things,” said Bhattacharya, a member of the UArizona Cancer Center and the BIO5 Institute who also serves on the UArizona Health Sciences Center for Molecular and Immunological Therapies advisory council.
“Transplantation across species, across the xenogeneic barrier, is difficult and is a very high bar for transplantation. We decided if we could overcome that barrier, then we could start to have confidence that we can overcome what should be a simpler human-to-human barrier, and so that’s basically what we did.”
The research team tested the modified stem cells by placing them into mice with normal, fully functioning immune systems. The results were promising—the genetically engineered pluripotent stem cells were integrated and persisted without being rejected.
“That has been the holy grail for a while. You might actually have a chance of being able to perform pluripotent stem cell-based transplants without immune suppressing the person who is receiving them. That would be an important advance, both clinically and from the simple standpoint of scale,” Bhattacharya said. “You wouldn’t have to make individualized therapies for every single person—you can start with one pluripotent stem cell type, turn it into the cell type you want and then give it to almost anyone.”
The next steps, Bhattacharya said, include testing the genetically modified pluripotent stem cells in specific disease models. He is already working with collaborators at The New York Stem Cell Foundation and the Juvenile Diabetes Research Foundation to test the technology in animal models for Type 1 diabetes.
“We needed to overcome the immune system first. The next steps are how do we use these cells?” Bhattacharya said. “We set the bar pretty high for our study and the fact that we were successful gives us some confidence that this can really work.”
More information: Hannah A. Pizzato et al, Engineering human pluripotent stem cell lines to evade xenogeneic transplantation barriers, Stem Cell Reports (2024). DOI: 10.1016/j.stemcr.2023.12.003
Journal information: Stem Cell Reports
Provided by University of Arizona

Leave a Reply