by Ingrid Fadelli , Medical Xpress
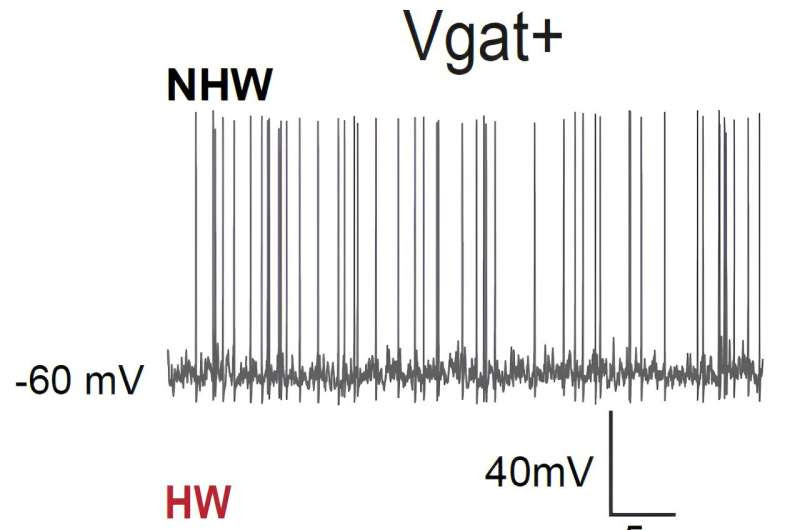
The medial preoptic area mediates depressive-like behaviors associated with ovarian hormone fluctuations Electrophysiological recording from MPOA GABAergic neurons (Vgat+) demonstrated that these neurons reduced their spiking activity in HW-treated mice compared to non-HW controls (NHW). Credit to Can Tao.
Fluctuations in the hormones secreted by women’s ovaries, namely estrogen and progesterone, are known to cause mood swings. For instance, at different points of the menstrual cycle, during or after pregnancy and while reaching menopause, women can experience lethargy, sadness, irritability, or other emotional changes.
In some cases, changes in the secretion of ovarian hormones can cause depressive-like symptoms. Two key examples of this are postpartum and perimenopausal depression, both of which are associated with the uneven rise and fall of female hormones secreted by the ovaries.
Researchers at University of Southern California recently carried out a study investigating the neural mechanisms through which these hormonal transitions lead to depressive-like symptoms. Their findings, published in Nature Neuroscience, highlight the role of the medial preoptic area (MPOA), part of the hypothalamus, in mediating female hormone-related depressive-like behaviors.
“Two years ago, we published a Nature Neuroscience paper, where we found that MPOA glutamatergic neurons mediate stress-induced anxiety,” Huizhong W. Tao, one of the researchers who carried out the study, told Medical Xpress. “This result inspired us to explore whether the MPOA can play a more general role in emotional/mood regulation.”
The key objective of the recent work by Tao, Zhang and their colleagues was to determine whether neurons in the MPOA also play a part in depressive states that are sometimes linked with fluctuations in ovarian hormones. To do this, the team carried out a series of experiments on female mice.
The first of these experiments entailed recording cell-specific neural activity in the MPOA of female mice in vivo (i.e., in live animals). They found that when these mice were experiencing so-called ovarian hormone withdrawal (HW), which essentially means that the levels of progesterone and estrogen in their body dropped rapidly, they tended to exhibit depressive-like behaviors.
“We found that in these depressive mice, GABAergic (but not glutamatergic) neurons in MPOA reduced their baseline activity compared to non-HW controls,” Tao explained. “We then manipulated activity levels of the GABAergic neurons using optogenetics or chemogenetics. We found that artificially increasing activity of the GABAergic neurons in MPOA of HW-treated mice alleviated the depressive state, while decreasing their activity in non-treated mice induced depressive-like behaviors.”
Overall, the researchers found there was a strong correlation between the activity level of MPOA GABAergic neurons (particularly estrogen receptor 1 expressing neurons) in the female mice brain and the manifestation of depressive states. This suggests that the MPOA may play a part in the depression-like symptoms that female mammals sometimes experience at different points of the menstrual cycle, around menopause and after giving birth.
“Our study pinpoints a specific neuronal type (estrogen receptor 1 expressing GABAergic neurons) in a specific brain area (MPOA) to account for depressive states associated with ovarian hormone fluctuations,” Tao said. “This finding has a strong implication for developing treatments for postpartum and perimenopausal depression, which are linked to hormone fluctuations in women.”
The results gathered by Tao, Zhang and their colleagues could pave the way for new studies further examining the specific processes through which MPOA neurons, particularly the specific type of GABAergic neurons they identified, mediate ovarian HW-related depressive-like states. Ultimately, this could lead to the development of more effective therapeutic interventions for various mental health disorders, including postpartum and perimenopausal depression, as well as premenstrual dysphoric disorder (PMDD).
“In our next studies, we plan to investigate whether the glutamatergic neurons in MPOA can also play a role in depressive states induced by conditions other than ovarian hormone withdrawal, for example, by early life stress,” Tao added.
More information: Can Tao et al, The medial preoptic area mediates depressive-like behaviors induced by ovarian hormone withdrawal through distinct GABAergic projections, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01397-2.
Journal information: Nature Neuroscience
© 2023 Science X Network

Leave a Reply