by UT Southwestern Medical Center
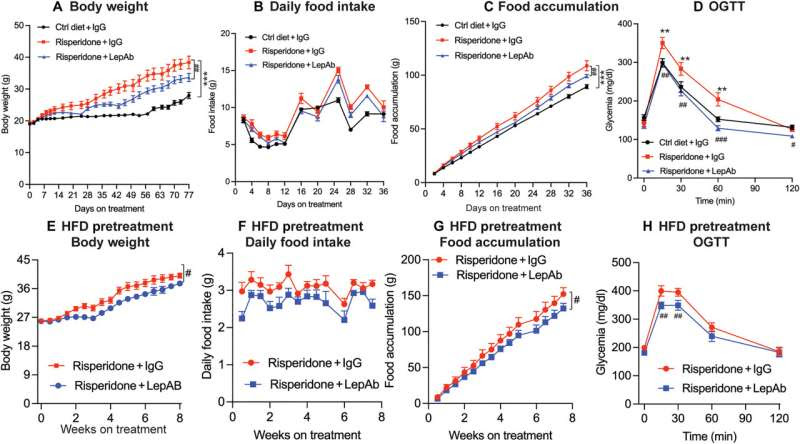
Effect of risperidone and risperidone + LepAb on body weight, food intake, and glucose tolerance. Female mice (n = 9) were placed on an HFD alone (Ctrl) or an HFD supplemented with risperidone. Mice were treated with either control IgG or LepAb twice weekly. Body weight and food intake were measured. Glucose tolerance was measured by a GTT at the end of the experiment. (A) Body weight; (B) daily food intake; (C) cumulative food intake; (D) glucose tolerance. A second cohort of female mice (n = 8 per group) was placed on an HFD plus risperidone diet for 4 weeks to achieve substantial obesity. The mice were then injected with IgG or LepAb for another 8 weeks. (E) Body weight; (F) daily food intake; (G) food accumulation; (H) oral glucose tolerance test (OGTT). Credit: Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.ade8460
An increased concentration of the hormone leptin in fat cells is believed to be responsible for weight gain associated with antipsychotic drugs, according to research led by UT Southwestern Medical Center. The study, published in Science Translational Medicine, used a mouse model to uncover the underlying mechanisms of unwanted metabolic side effects and to test an antibody that might reduce them.
Despite the effectiveness of antipsychotic drugs in managing psychiatric conditions, prescriptions such as olanzapine and risperidone can cause patients to add significant weight and in some cases result in diabetes and liver disease.
“Weight gain affects most patients who start taking these antipsychotic drugs and is a well-established side effect of these interventions. As a result, many individuals become insulin resistant and diabetic. The study implicates leptin as a key driver of these negative metabolic consequences,” said Philipp Scherer, Ph.D., Professor of Internal Medicine and Director of the Touchstone Center for Diabetes Research at UT Southwestern.
Patients using antipsychotic drugs experience increased concentrations of circulating leptin released from fat cells before they begin to gain weight. Prior to this study, however, it was not well understood how increased leptin, or hyperleptinemia, was connected to this drug-induced weight gain.
“While it was historically viewed as a ‘passenger’ to obesity—meaning levels go up as we gain weight—our data strongly suggest that it is a ‘driver’ for drug-induced obesity,” Dr. Scherer explained.
Using a previously tested mouse model, researchers obtained evidence that hyperleptinemia was directly contributing to obesity and issues such as liver fibrosis, insulin resistance, and untimely mammary duct development. Moreover, researchers found that hyperleptinemia increased inflammation, which is considered a main contributor in the development of antipsychotic drug-induced metabolic disorders.
Results also indicated that treating mice with a leptin-neutralizing antibody greatly reduced the side effects. Treatment with the antibody led to reduced weight gain, inflammation, and mammary gland development as well as improved glucose tolerance in mice with drug-induced side effects. These key findings provide a path toward improving outcomes for patients in the future.
“We are working hard to take the leptin-neutralizing antibodies that we have used here into a clinical setting to find out whether the mechanisms defined in rodents also apply to individuals who embark on an antipsychotic treatment regimen,” Dr. Scherer noted.
More information: Shangang Zhao et al, Hyperleptinemia contributes to antipsychotic drug–associated obesity and metabolic disorders, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.ade8460
Journal information: Science Translational Medicine
Provided by UT Southwestern Medical Center

Leave a Reply