September 9, 2024
by Genomic Press
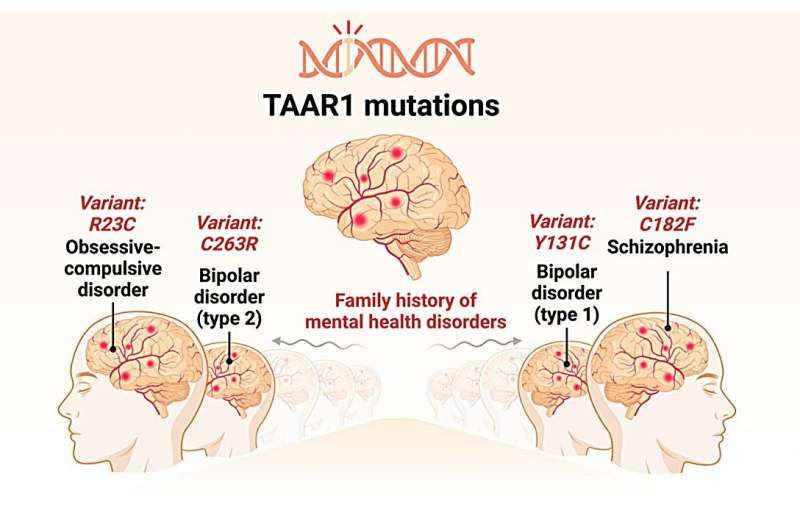
The association between TAAR1 variants, neuropsychiatric disorders, and therapeutic response. TAAR1 mutations (only selected mutations are shown for clarity) in patients with neuropsychiatric disorders (top panel). A model proposing altered signaling of trace amines in variant TAAR1 observed in neuropsychiatric disorders (bottom left). Treatment options are tailored to patients with TAAR1 mutations (bottom right). The figure was created with Biorender.com. Credit: Genomic Psychiatry (2024). DOI: 10.61373/gp024v.0058
In the complex landscape of mental health research, a new viewpoint review sheds light on an underexplored genetic player: the trace amine-associated receptor 1 (TAAR1). Published in Genomic Psychiatry, this analysis suggests that mutations in the TAAR1 gene may be a crucial piece of the puzzle in understanding and treating neuropsychiatric disorders.
TAAR1, primarily expressed in the brain, has recently caught the attention of neuroscientists and pharmaceutical companies alike. It’s now considered a promising target for treating conditions like schizophrenia, with several TAAR1-focused drugs in clinical trials. However, the potential role of TAAR1 genetic variations in mental health disorders has remained largely unexplored—until now.
“We’re seeing intriguing connections between rare TAAR1 mutations and various psychiatric conditions,” explains lead author and a Ph.D. candidate Britto Shajan of Flinders University. “These genetic variants seem to alter the receptor’s function, potentially contributing to the development of mental health disorders.”
The review, co-authored by experts from Flinders Health and Medical Research Institute, Flinders University and Monash University in Australia, synthesizes findings from several clinical studies that have identified rare TAAR1 mutations in patients with schizophrenia, bipolar disorder, and other psychiatric conditions. Key insights include:
Some TAAR1 variants significantly impair the receptor’s function, disrupting important brain signaling pathways.
There may be a link between TAAR1 mutations, cognitive function, and metabolic disorders, hinting at complex mind-body interactions.
TAAR1 genetic variations could influence how patients respond to emerging TAAR1-targeted therapies.
“This review opens up exciting new avenues for research,” says senior author Dr. Pramod C. Nair. “Understanding how TAAR1 variants affect brain function could lead to more personalized treatment approaches. We might even see genetic testing for TAAR1 mutations becoming part of psychiatric care in the future.”
The authors emphasize that while current findings are promising, larger studies are needed to fully grasp the significance of these rare genetic variants. They also highlight the potential for TAAR1 mutations to affect interactions with other neurotransmitter systems, such as dopamine signaling—a key player in many psychiatric disorders.
This fresh perspective on TAAR1 genetics comes at a pivotal time in mental health research. Previous studies have shown altered levels of trace amines (the molecules that activate TAAR1) in patients with various brain disorders. However, the exact mechanisms linking these changes to psychiatric symptoms have remained elusive.
“Our review suggests that genetic variations in TAAR1 could be a missing link in understanding how trace amine imbalances contribute to mental health disorders,” explains co-author Professor Tarun Bastiampillai. “This could have profound implications for drug development and personalized medicine in psychiatry.”
The authors call for further research to explore how TAAR1 mutations might affect drug efficacy and side effects. They suggest that future development of TAAR1-targeted therapies should consider the potential impact of genetic variations.
“As we move towards precision psychiatry, understanding the genetic underpinnings of these disorders becomes increasingly crucial,” adds Dr. Nair. “Our review highlights TAAR1 as a potentially important piece of this complex puzzle.”
This comprehensive analysis of TAAR1 genetic variants and their possible role in neuropsychiatric disorders represents a significant step towards unraveling the intricate genetic architecture of mental health conditions. As research in this area advances, it may open new doors for diagnosis, treatment, and even prevention of these challenging disorders.
More information: The association between trace amine-associated receptor 1 (TAAR1) genetic mutations and neuropsychiatric disorders, Genomic Psychiatry (2024). DOI: 10.61373/gp024v.0058. gp.genomicpress.com/wp-content … GP0058-Nair-2024.pdf
Provided by Genomic Press

Leave a Reply