Business Announcement INSILICO MEDICINEAMYOTROPHIC LATERAL SCLEROSIS (ALS) IS A FATAL TYPE OF MOTOR NEURON DISEASE CHARACTERIZED BY PROGRESSIVE DEGENERATION OF NERVE CELLS IN THE SPINAL CORD AND BRAIN. CREDIT: INSILICO MEDICINE Two years after the collaboration was announced between Insilico and 4B Technologies, the AI-discovered ALS drug, FB1006, completed patient enrollment for the IIT study.From...
Tag: <span>ALS drug</span>
China’s experimental ALS drug SNUG01 has unexpected results for patient, may be useful for Parkinson’s, Alzheimer’s, stroke victims
A potential new treatment for neurological disease ALS has shown promising results after being tested on its first patientALS is notoriously difficult to treat, with most patients dying three to five years after the onset of symptomsDannie PengIn the legendary life of world-renowned British physicist Stephen Hawking, among his many “achievements” was the fact that...
Clinical trial suggests ALS drug effective for treating spinal cord injuries
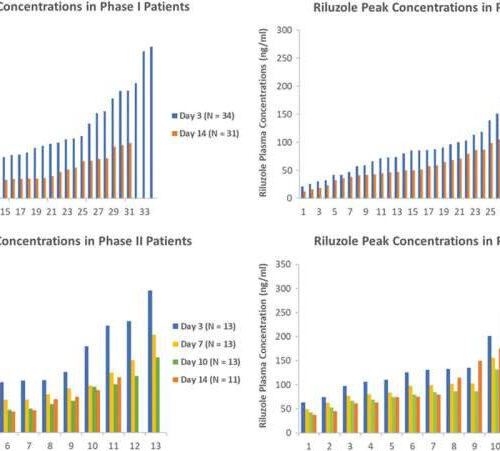
by Laurie Fickman, University of Houston Riluzole trough and peak concentrations from Phase I and Phase II/III trials presented in waterfall plots. Credit: Journal of Neurotrauma (2023). DOI: 10.1089/neu.2022.0499 A small clinical trial with a pharmacokinetic sub-study, led by a pharmacologist at the University of Houston, has demonstrated the promising effectiveness of the drug Riluzole for...
Hotly anticipated ALS drug could pave way for more brain treatments
Asher Mullard In people with amyotrophic lateral sclerosis (ALS), motor neurons, which help to send commands from the brain to muscle cells, become damaged, as depicted in this artist rendering.Credit: Kateryna Kon/Science Photo Library The US Food and Drug Administration (FDA) is set to rule soon on the approval of a new drug for a...
ALS drug wins FDA approval despite questionable data
by MATTHEW PERRONE Credit: Unsplash/CC0 Public Domain A much-debated drug for Lou Gehrig’s disease won U.S. approval Thursday, a long-sought victory for patients that is likely to renew questions about the scientific rigor behind government reviews of experimental medicines. The Food and Drug Administration approved the drug from Amylyx Pharmaceuticals based on results from one small, mid-stage...
Experimental ALS drug may be more effective than existing ones
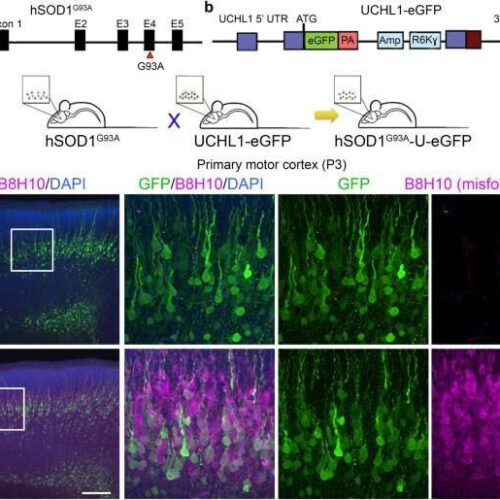
by Northwestern University When mixed cortical cultures are established at P3, UMNs are labeled by eGFP expression in UCHL1-eGFP reporter mice, and express disease-causing proteins in reporter lines of mouse models of amyotrophic lateral sclerosis (ALS) (a-c) Generation of hSOD1G93A-UeGFP and WT-UeGFP littermate control mice. (d-e) UMNs in layer 5 of the primary motor cortex...
FDA approves oral formulation of old ALS drug, giving patients new treatment option in sorely needed field
Max Gelman Senior Editor Much of the ALS drug news in recent months has focused on Amylyx Pharmaceuticals and its experimental program reviewed by an FDA advisory committee in March. But on Thursday, the FDA greenlighted a new way to take an already-approved ALS drug that flew under the radar. US regulators OK’ed an oral...
Biogen reverses course on compassionate use for ALS drug tofersen with plans to open program in July
Josh Sullivan Associate Editor Despite immense pressure from the ALS community, Biogen had repeatedly refused to open investigational drug tofersen for use outside of clinical trials. But now, caving to that demand, Biogen has reversed its stance. With a pivotal Phase III study in ALS ongoing and data expected to read out later this year, Biogen just...