by Melody Petersen, Los Angeles Times Credit: Pixabay/CC0 Public Domain For those fearing their memories are fading, the ads provide hope. “Early diagnosis extends quality of life,” says an ad by Dung Trinh, an Orange County physician, on the website of a program for seniors at Mount of Olives Church in Mission Viejo. Trinh offers...
Tag: <span>Alzheimer’s drug</span>
It’s big-time progress:’ Groundbreaking Alzheimer’s drug soon to gain FDA approval
Updated: Jul. 03, 2023, 1:56 p.m. Published: Jul. 03, 2023, 5:30 a.m. Leqembi, a monoclonal antibody manufactured by Eisai and Biogen, is the first to reduce the amount of amyloid beta plaque in the brain, slowing down the progression of the illness. Though it can’t reverse the damage already done by Alzheimer’s disease, it can produce...
FDA-approved Alzheimer’s drug lecanemab could prevent free-floating amyloid beta fibrils from damaging the brain
by Cell Press Credit: Pixabay/CC0 Public Domain For the first time, researchers described the structure of a special type of amyloid beta plaque protein associated with Alzheimer’s disease (AD) progression. In a report published May 10 in the journal Neuron, scientists showed the small aggregates of the amyloid beta protein could float through the brain tissue fluid, reaching many...
Clinical trial participant’s autopsy and brain exam stoke Alzheimer’s drug fears
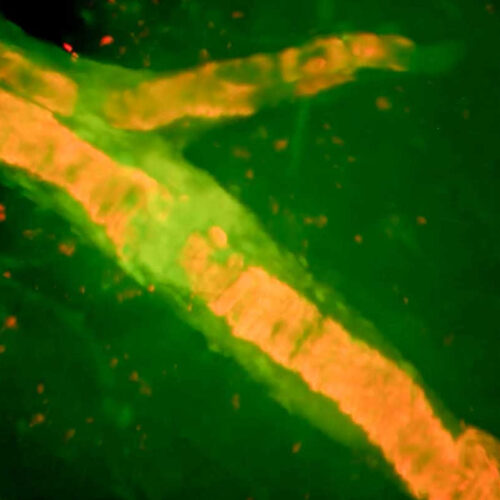
13 APR 2023 8:00 AM BY CHARLES PILLER The brain of a woman who died after receiving a new Alzheimer’s disease drug shows amyloid (orange) lining blood vessels and a site where a vessel ruptured and bled (yellow-green). LISSA VENTURA-ANTUNES A full autopsy and detailed examination of the brain of a 79-year-old Florida woman who...
Alzheimer’s drug saga prompts journal to scrutinize whistle-blowers
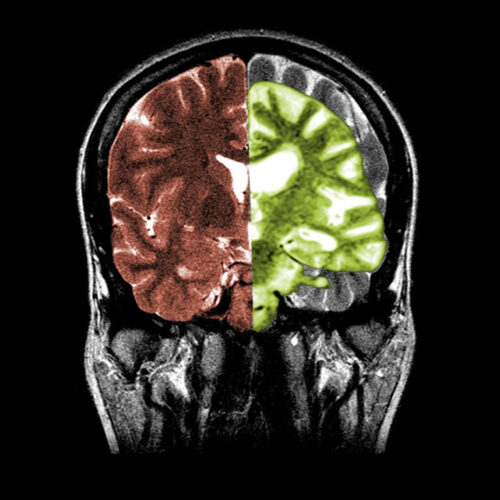
Holly Else Alzheimer’s disease, as shown on the right side of this scan, transforms the brains of people with the neurodegenerative condition.Credit: Science History Images/Alamy A scientific journal has revamped its whistle-blower policy amid a dispute over the integrity of research underlying an experimental Alzheimer’s drug. In a 1 November 2022 editorial in The Journal of Clinical Investigation (JCI), editor-in-chief Elizabeth McNally...
Scientists tie third clinical trial death to experimental Alzheimer’s drug
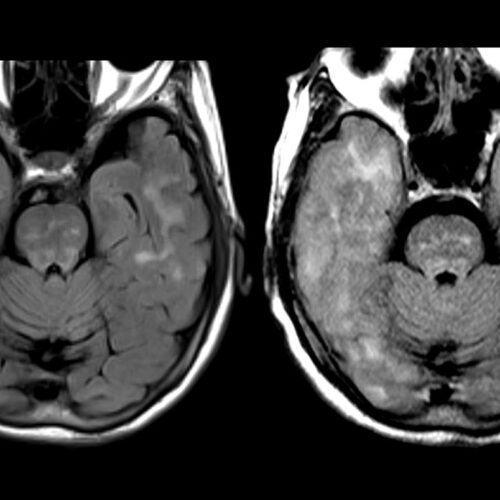
BY CHARLES PILLER These MRI images show brain swelling in the cerebral cortex—the outer large section of the brain—of a Florida woman who died after receiving the experimental Alzheimer’s drug lecanemab. Before treatment with the antibody, a scan of her cerebral cortex (left) reveals characteristic folds of the temporal lobes. Afterward (right), extreme swelling made...
Is lecanemab the Alzheimer’s drug that will finally make a difference?
December 8, 2022 6:00 AM ET JON HAMILTON In a large study, experimental drug lecanemab was able to slow down Alzheimer’s, but not stop it. Some researchers think the drug will become the first to help many patients; others have questions. Cemile Bingol/Getty Images A drug that offers a small benefit to Alzheimer’s patients is...
New Alzheimer’s drug faces uncertain regulatory path
Caitlin Owens Oriana Gonzalez Illustration: Aïda Amer/Axios Researchers have at last found a drug that can slow the progression of Alzheimer’s disease, according to clinical trial data presented last night. But regulators now have to weigh its relatively modest efficacy against safety risks. What we’re watching: The FDA will soon decide whether to approve Eisai’s experimental...
Alzheimer’s drug candidate may have potential for treating Parkinson’s disease
disease A recent study showed that itanapraced disrupted genetic mutations associated with Parkinson’s disease and reduced loss of neurons in cell culture and mouse models. ImagesBazaar/Getty Images Mutations that enhance the activity of the LRRK2 enzyme are one of the most common mutations in inherited and sporadic Parkinson’s disease. A recent study showed that mutations...
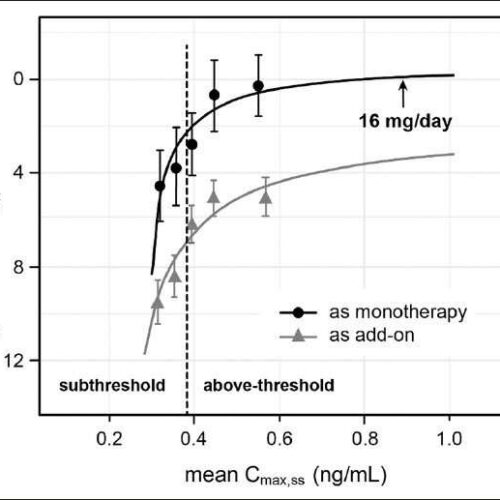
Alzheimer’s drug trial shows ‘evidence of sustained improvement’
by University of Aberdeen Relationship between steady-state plasma levels of hydromethylthionine and decline on the ADAS-Cog11 scale over 65 weeks in 566 participants receiving hydromethylthionine mesylate at 8 mg/day (100 as monotherapy, 466 as add-on to standard AD symptomatic drugs) in completed Phase 3 trials TRx-237-015 and TRx-237-005). Patient groups with subthreshold and above-threshold exposure to...