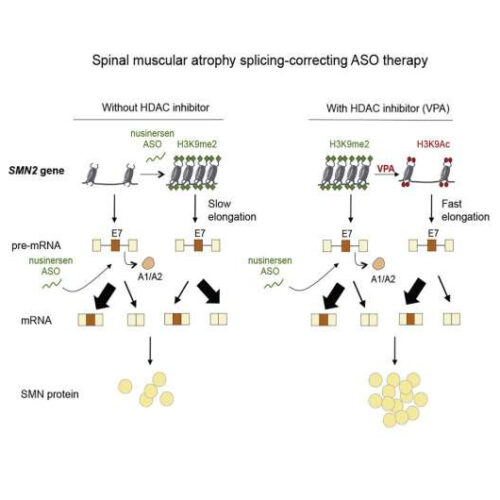
by Cold Spring Harbor Laboratory Graphical abstract. Credit: Cell (2022). DOI: 10.1016/j.cell.2022.04.031 In 2016, Spinraza became a game-changer for spinal muscular atrophy (SMA) patients. It was the first FDA-approved treatment for the neurodegenerative disease, which is the leading genetic cause of infant death. The drug was conceived and developed by Cold Spring Harbor Laboratory (CSHL) Professor Adrian Krainer and collaborators. But Krainer...