by Physician’s Briefing Staff After approving Casgevy (exagamglogene autotemcel) in December to treat sickle cell disease, the U.S. Food and Drug Administration announced Tuesday that the therapy has now been approved to treat patients older than 12 years with transfusion-dependent beta-thalassemia. Casgevy is the first CRISPR-based medicine, where gene editing is used to develop the...
Tag: <span>beta-thalassemia</span>
Post
Gene therapy promotes transfusion independence for severe beta-thalassemia
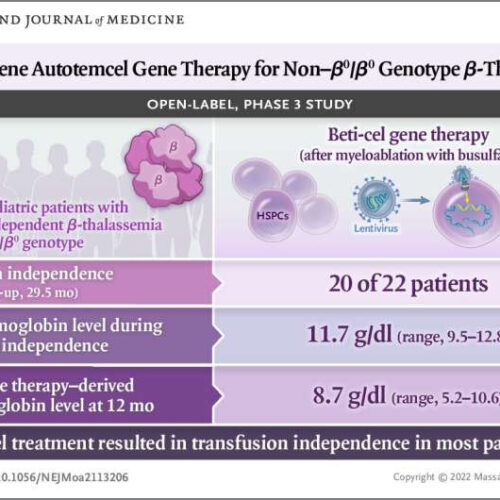
by Melissa Rohman, Northwestern University Graphical abstract. Credit: DOI: 10.1056/NEJMoa2113206 A novel gene therapy promoted transfusion independence in more than 90 percent of adult and pediatric patients with transfusion-dependent beta-thalassemia, according to a recent clinical trial published in the New England Journal of Medicine. The therapy represents a potentially curative treatment option for patients who must...