by Elana Gotkine CD19 chimeric antigen receptor (CAR) T-cell therapy seems feasible, safe, and efficacious for patients with different autoimmune diseases, according to a study published in the Feb. 22 issue of the New England Journal of Medicine. Fabian Müller, M.D., from the Friedrich-Alexander University Erlangen-Nürnberg in Germany, and colleagues examined patients with severe systemic...
Tag: <span>CAR-T cel therapy</span>
Post
FDA warns of rare secondary cancer risk with CAR-T therapies
by Robin Foster The U.S. Food and Drug Administration has told drugmakers to add a boxed warning to a type of cancer treatment called CAR-T therapy, saying the treatment itself may sometimes cause a secondary cancer. Still, FDA spokesperson Carly Kempler told NBC News that, despite the new warning, “the overall benefits of these products...
Post
CAR T-cell therapy effective in patients with blood cancer regardless of race: Study
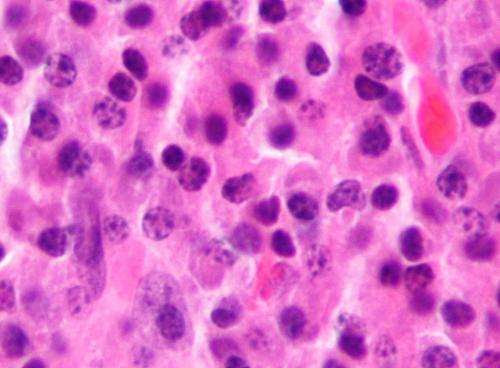
by American Society of Hematology Micrograph of a plasmacytoma, the histologic correlate of multiple myeloma. H&E stain. Credit: Wikipedia/CC BY-SA 3.0Patients with multiple myeloma treated with idecabtagene vicleucel, known as “ide-cel,” a chimeric antigen receptor (CAR) T-cell therapy, had no difference in overall survival outcomes regardless of race and ethnicity, according to a study published in...