by Keila DePape, McGill University Credit: Life Science Alliance (2024). DOI: 10.26508/lsa.202402831 A novel drug holds promise for treating Duchenne muscular dystrophy (DMD), a rare genetic disorder that causes severe muscle degeneration. McGill University researchers have discovered that an experimental compound called K884 can boost the natural repair abilities of muscle stem cells. Current treatments can slow muscle damage,...
Tag: <span>Duchenne Muscular Dystrophy</span>
Experts assess promise of vamorolone to treat Duchenne muscular dystrophy
Commentary in the Journal of Neuromuscular Diseases provides an independent, unbiased assessment of the body of research on vamorolone Peer-Reviewed Publication IOS PRESS The drug vamorolone (Agamree®) has been hailed as a promising new drug to treat Duchenne muscular dystrophy (DMD). It has recently been approved for clinical use in the United States by the...
FDA Approves First Gene Therapy for Treatment of Certain Patients with Duchenne Muscular Dystrophy
June 22, 2023 Today, the U.S. Food and Drug Administration approved Elevidys, the first gene therapy for the treatment of pediatric patients 4 through 5 years of age with Duchenne muscular dystrophy (DMD) with a confirmed mutation in the DMD gene who do not have a pre-existing medical reason preventing treatment with this therapy. “Today’s approval addresses...
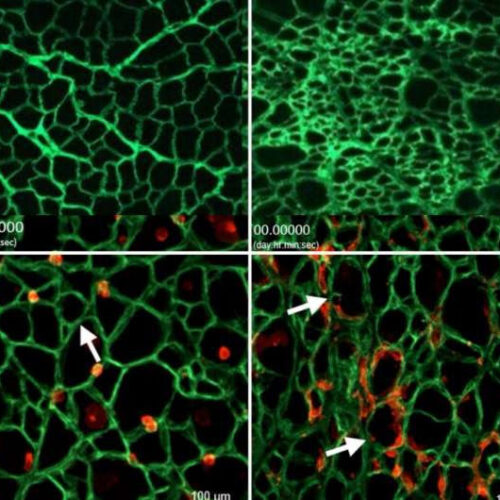
Study: Scarring of collagen ‘highway’ prevents stem cells from healing damaged tissue in Duchenne muscular dystrophy
by Holly Ober, University of California, Los Angeles Top, left: A healthy myoscaffold. Top right: A Duchenne muscular dystrophy scaffold. Bottom left: Stem cells (red) growing in a healthy myoscaffold (green). Bottom right: Stem cells growing in a Duchenne myoscaffold (green). Credit: Rachelle Crosbie Muscles that ache after a hard workout usually don’t hurt for long,...
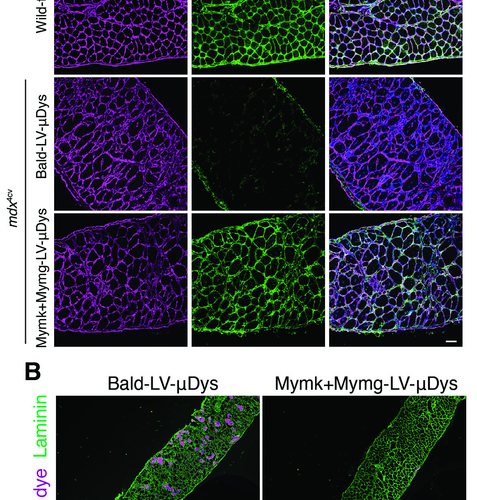
Bold new therapy delivery method shows initial promise as treatment for Duchenne muscular dystrophy
CINCINNATI CHILDREN’S HOSPITAL MEDICAL CENTER IMAGE: RESEARCHERS SHOWED THAT SYSTEMIC DELIVERY OF MYMK+MYMG-LV-DYS RESULTED IN A SIGNIFICANT LEVEL OF DYS+ MYOFIBERS. IMPORTANTLY, THE TEAM DID NOT DETECT EVIDENCE OF VIRAL TRANSDUCTION IN NON-SKELETAL MUSCLE TISSUES INCLUDING HEART, KIDNEY, LIVER, AND SPLEEN. DYSTROPHIC MICE EXHIBITED HEALTHIER MUSCLES (REDUCTIONS IN MUSCLES CELLS WHERE A DYE WAS ABLE...
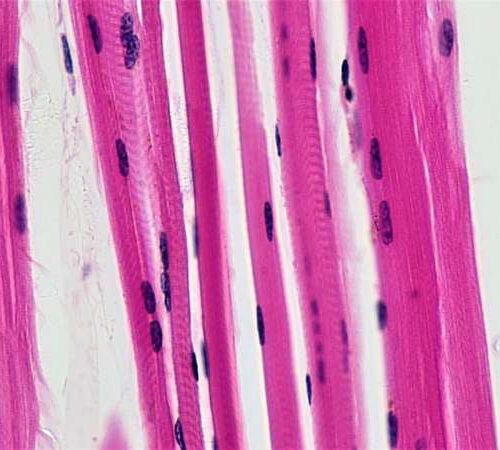
Study finds daily steroids safe and slow the progression of Duchenne muscular dystrophy
by Mark Michaud, University of Rochester Medical Center Skeletal muscle fibers. Credit: Berkshire Community College Bioscience Image Library / Public domain New research published in JAMA recommends daily steroid doses for children with Duchenne muscular dystrophy (DMD), marking a significant change in how the disease is treated. University of Rochester Medical Center (URMC) neurologist Robert Griggs, M.D.,...
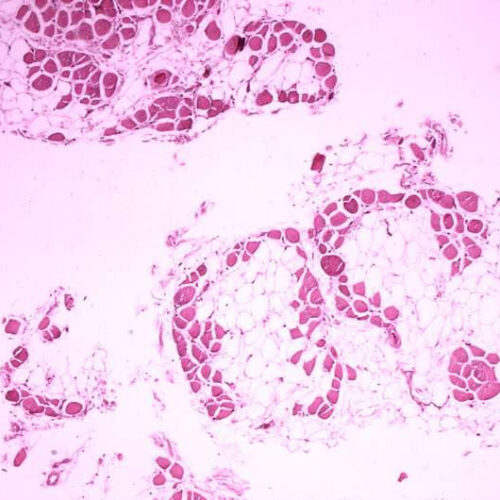
Piezo1 possible key to supporting muscle regeneration in Duchenne Muscular Dystrophy
by Perelman School of Medicine at the University of Pennsylvania Histopathology of gastrocnemius muscle from patient who died of pseudohypertrophic muscular dystrophy, Duchenne type. Cross section of muscle shows extensive replacement of muscle fibers by adipose cells. Credit: Public Domain One protein, Piezo1, is key to marshalling muscle stem cells’ unique shapes and response to...
New discovery could lead to therapies for patients with Duchenne muscular dystrophy
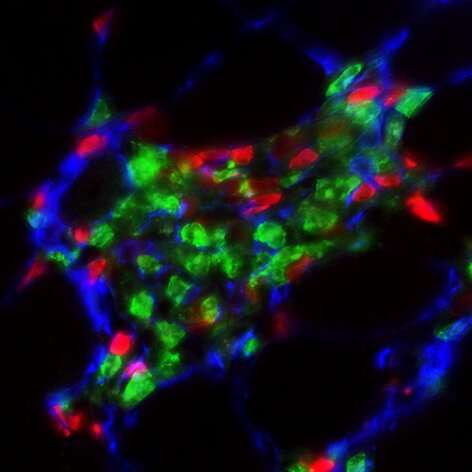
by University of California, Irvine The analysis of dystrophic quadriceps by immunofluorescence microscopy highlights a novel interaction between immune cells and stromal progenitors that stimulates fibrosis during muscular dystrophy. Eosinophils are depicted in green, ILC2s in red and stromal progenitors in blue. Credit: UCI School of Medicine A new study, led by the University of California,...
FDA OKs Golodirsen (Vyondys 53) for Duchenne Muscular Dystrophy
Megan Brooks The US Food and Drug Administration (FDA) has approved golodirsen injection (Vyondys 53, Sarepta Therapeutics), the first treatment for Duchenne muscular dystrophy (DMD) in patients with a confirmed mutation amenable to exon 53 skipping. Last summer, the agency declined to approve of the drug on an accelerated basis, sending a complete response letter...
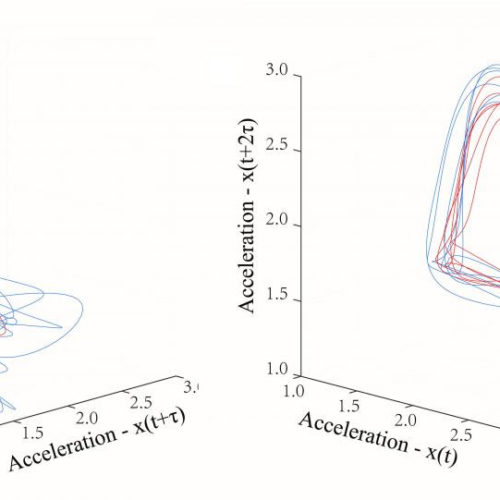
Duchenne muscular dystrophy diagnosis improved by simple accelerometers
As the most common form of the disease, early diagnosis of Duchenne muscular dystrophy is key to survival AMERICAN INSTITUTE OF PHYSICS WASHINGTON, February 11, 2020 — Duchenne muscular dystrophy is the most common type of muscular dystrophy, affecting more than 10,000 males at birth per year in the United States with severe physical disability,...
- 1
- 2