by Purdue University R. Claudio Aguilar, a Purdue University researcher, has developed a patented therapeutic strategy for Lowe syndrome by repurposing two drugs previously approved by the U.S. Food and Drug Administration. Credit: Purdue University College of Science photo/Alisha ReferdaPurdue University researchers have developed a patented therapeutic strategy for Lowe syndrome, an incurable and rare genetic...
Tag: <span>fda approved</span>
Alzheimer’s blood test found to perform as well as FDA-approved spinal fluid tests
by Washington University School of Medicine Credit: Unsplash/CC0 Public DomainA simple blood test to diagnose Alzheimer’s disease soon may replace more invasive and expensive screening methods such as spinal taps and brain scans. A study by researchers at Washington University School of Medicine in St. Louis and Lund University in Sweden shows that a blood test...
FDA approves exagamglogene autotemcel to treat beta-thalassemia
by Physician’s Briefing Staff After approving Casgevy (exagamglogene autotemcel) in December to treat sickle cell disease, the U.S. Food and Drug Administration announced Tuesday that the therapy has now been approved to treat patients older than 12 years with transfusion-dependent beta-thalassemia. Casgevy is the first CRISPR-based medicine, where gene editing is used to develop the...
Caltech Startup Receives FDA Clearance for Heart Health Diagnostic
Posted Today Heart failure is one of the leading causes of hospitalization among older adults in the United States, with over half a million new cases being recorded each year. Half of these individuals will die within five years of developing the disease.An artist’s rendering of a heartbeat plotted on a graph. An artist’s rendering of...
FDA approves Takeda’s new enzyme replacement therapy for rare blood clotting disorder
Christophe Weber, Takeda CEO Shoko TakayasuNovember 9, 2023FDA approves Takeda’s new enzyme replacement therapy for rare blood clotting disorderZachary BrennanSenior EditorThe FDA on Thursday approved Takeda’s Adzynma, an enzyme replacement therapy for adult and pediatric patients with congenital thrombotic thrombocytopenic purpura (cTTP), which is a rare and potentially fatal blood clotting disorder. The approval was...
FDA approves Wezlana for multiple inflammatory diseases
by Lori Solomon The U.S. Food and Drug Administration has approved Amgen’s Wezlana (ustekinumab-auub) as a biosimilar to and interchangeable with Stelara (ustekinumab) for multiple inflammatory diseases. The approval includes indications for adults with moderate-to-severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderately to severely active Crohn disease, and...
FDA approves mirikizumab, a promising induction and maintenance therapy for ulcerative colitis
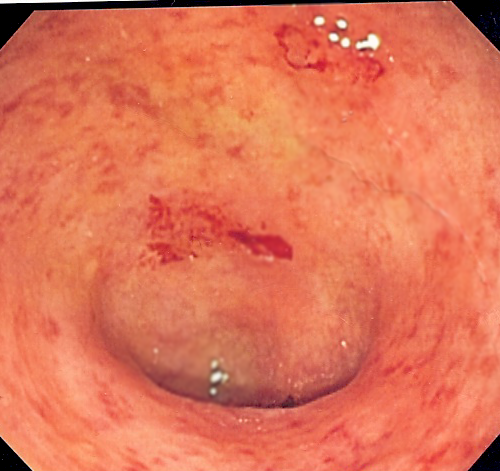
by The Mount Sinai Hospital Endoscopic image of a bowel section known as the sigmoid colon afflicted with ulcerative colitis. The internal surface of the colon is blotchy and broken in places. Credit: Samir/Wikipedia The U.S. Food and Drug Administration (FDA) approved mirikizumab, on October 26, 2023, a highly effective new treatment for ulcerative colitis (UC),...
FDA approves muscular dystrophy drug built on Children’s National research
Vamorolone offers new treatment option with fewer side effects Business Announcement CHILDREN’S NATIONAL HOSPITAL WASHINGTON (October 27, 2023) – Boys with Duchenne muscular dystrophy (DMD) have a clinically proven, new treatment option with the Food and Drug Administration’s approval Thursday of vamorolone, a steroidal-type, anti-inflammatory drug developed based on research performed at Children’s National Hospital. Created...
FDA approves Zilbrysq for generalized myasthenia gravis
by Lori Solomon The U.S. Food and Drug Administration has approved UCB’ Zilbrysq (zilucoplan) for the treatment of adults with generalized myasthenia gravis. Zilbrysq is a targeted peptide inhibitor of complement component 5 (C5) and is approved as a once-daily, self-administered, targeted therapy in patients with anti-acetylcholine receptor (AChR)-antibody-positive generalized myasthenia gravis. The approval of...
EXCLUSIVE: Constipation pill that VIBRATES as it moves through the body could be remedy for America’s laxative shortage – and it’s already approved by the FDA
Vibrant, is not a drug but a capsule that stimulates natural movements in the gut Its vibrations stimulate the colon to contract naturally to relieve constipation READ MORE: America suffering shortage of LAXATIVES due to surging demand By CAITLIN TILLEY, HEALTH REPORTER FOR DAILYMAIL.COM PUBLISHED: 11:48 EDT, 18 October 2023 | UPDATED: 11:56 EDT, 18 October 2023 An easy-to-swallow vibrating capsule that offers...