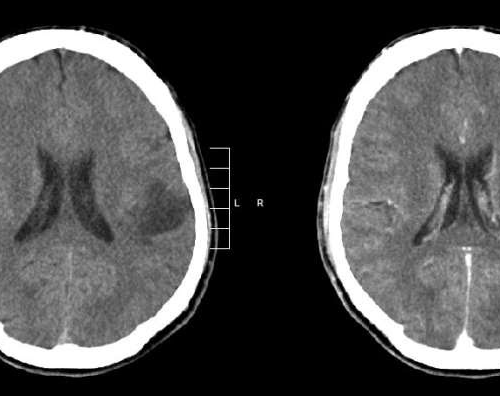
August 7, 2024 by Dana-Farber Cancer Institute Glioma of the left parietal lobe. CT scan with contrast enhancement. Credit: Mikhail Kalinin/CC BY-SA 3.0Vorasidenib has been approved by the U.S. Food and Drug Administration (FDA) for patients with Grade 2 gliomas with IDH1 or IDH2 mutations. Based on evidence from the INDIGO clinical trial, a global...