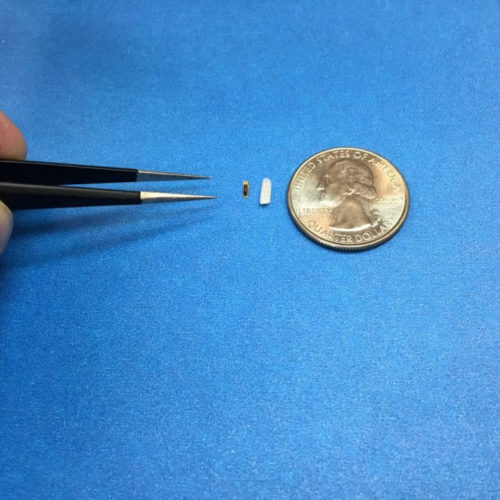
EMERYVILLE, Calif., September 1, 2020 — Injectsense, a company focused on sensor-enabled digital health, announced it has received a Breakthrough Device Program (BDP) designation from the U.S. Food and Drug Administration (FDA) for its chronic continuous IOP monitoring system for glaucoma patients. The company’s IOP Connect™ system is based on a long-term implantable sensor, smaller...