The US Food and Drug Administration (FDA) has approved the oral Janus kinase (JAK) inhibitor deuruxolitinib for the treatment of adults with severe alopecia areata. The development, which was announced in a July 25, 2024, news release from the drug’s manufacturer Sun Pharma, is based on data from two pivotal randomized, double-blind, placebo-controlled phase 3...
Tag: <span>JAK inhibitor</span>
Post
Treating sarcoidosis with JAK inhibitor shows promise in clinical trial
Every patient in a Yale clinical trial of a new treatment for the disfiguring disease sarcoidosis saw an improvement in their skin — and more than half showed improvement in affected internal organs. Sarcoidosis is a disease affecting four out of every 10,000 people in the United States. In sarcoidosis, abnormal collections of immune cells called granulomas...
Post
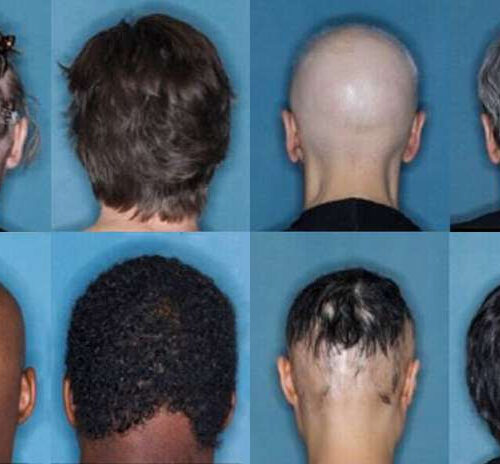
Latest JAK inhibitor trials for severe skin disease are a success
by Jim Shelton, Yale University Before and after images for participants who received 36 weeks of treatment for alopecia areata with baricitinib. Credit: Yale University A new study shows that one in three patients with a severe skin disease were able to regrow hair after being treated with a common arthritis drug. The study is...