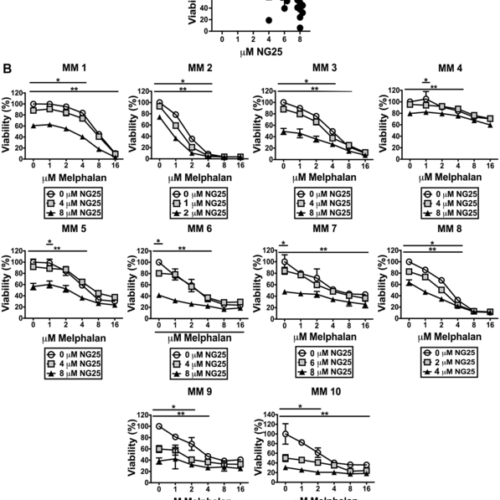
IMPACT JOURNALS LLC IMAGE: NG25 REDUCE VIABILITY OF CD138+ CELLS FROM PATIENTS, ALONE AND IN COMBINATION WITH MELPHALAN. (A) CD138+ CELLS FROM 10 INDIVIDUAL DONORS WERE TREATED WITH NG25 BEFORE CELL VIABILITY WAS MEASURED. INDIVIDUAL DONORS, MEAN, AND SD ARE SHOWN. ASTERISKS INDICATE STATISTICALLY SIGNIFICANT DIFFERENCES (KRUSKAL-WALLIS TEST, DUNN’S MULTIPLE COMPARISON TEST, P < 0,05)....
Tag: <span>multiple myeloma</span>
Researchers study potential new CAR-T cell therapy for multiple myeloma
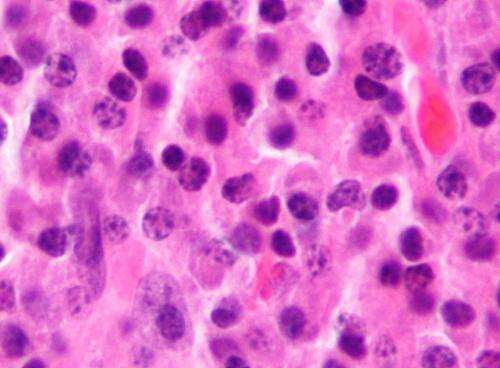
by Mayo Clinic Micrograph of a plasmacytoma, the histologic correlate of multiple myeloma. H&E stain. Credit: Wikipedia/CC BY-SA 3.0 Researchers at Mayo Clinic Cancer Center are studying a potential new chimeric antigen receptor-T cell therapy (CAR-T cell therapy) treatment for multiple myeloma. Their findings were published on Friday, June 24, in The Lancet. “CAR-T cell therapy is a type of...
New therapy shows promise in future treatment of multiple myeloma
by Peter MacCallum Cancer Centre Credit: National Cancer Institute \ Dana-Farber Harvard Cancer Center A preclinical study has found multiple myeloma is highly sensitive to a newly developed experimental therapy, according to research published today by Peter Mac scientists. Multiple myeloma is a type of blood cancer that originates in plasma cells, immune cells in the bone...
CAR T-cell therapy generates lasting remissions in patients with multiple myeloma
by Dana-Farber Cancer Institute Nikhil Munshi, MD Credit: Dana-Farber Cancer Institute In a major advance in the treatment of multiple myeloma, a CAR T-cell therapy has generated deep, sustained remissions in patients who had relapsed from several previous therapies, an international clinical trial has found. In a study posted online today by the New England Journal of...
FDA approves new multiple myeloma drug despite toxicity concerns
The Food and Drug Administration on Wednesday approved a new drug to treat patients with multiple myeloma, overruling a panel of outside cancer experts who expressed concerns about its toxicity. The new multiple myeloma drug, called selinexor, will be marketed by Karyopharm Therapeutics under the brand name Xpovio. The FDA cleared Xpovio under an accelerated,...
New Therapies Approved for Multiple Myeloma
Abstract and Introduction Abstract Multiple myeloma (MM) accounts for approximately 1% of all cancers and 10% of all hematologic malignant disorders. MM occurs slightly more often in men than in women, and it is twice as common in African American persons compared with white persons. The median age at diagnosis is approximately 65 years. Although...
Gene-Based Therapy That Reprograms Immune Cells Offers Hope For Multiple Myeloma Cure
Multiple Myeloma The United States has more than 30,000 cases of multiple myeloma occurring each year. The condition affects the plasma cells that produce antibodies that fight infection. Between 60,000 and 70,000 American currently have the disease. Only about half of these patients are expected to live five years after their diagnosis. Now, a gene-based...
Researchers ID drug that blocks some blood cancers
A compound identified by Weill Cornell Medicine scientists inhibits the growth of a rare blood cancer found in people with HIV-AIDS. Their research, published May 15 in the Journal of Clinical Investigation, also demonstrates that the compound deters multiple myeloma, another type of blood malignancy. Few effective treatments are available for primary effusion lymphoma(PEL), a subtype...
The evolution of proteasome inhibition: A personal journey from early research to approval
My 16 plus years at Takeda Oncology have given me the opportunity to participate in and contribute to the evolution of treatment for multiple myeloma, a rare cancer that affects nearly 230,000 people around the world, according to five-year prevalence estimates.1 While the disease remains incurable, we have made important advances in research and development in recent years....
Combo Rx plus stem-cell tx ups PFS in multiple myeloma
Michel Attal, M.D., from the Institut Universitaire du Cancer de Toulouse-Oncopole in France, and colleagues randomized patients with multiple myeloma to receive induction therapy with three cycles of RVD, then consolidation therapy with either five additional cycles of RVD (350 patients) or high-dose melphalan plus stem-cell transplantation followed by two additional RVD cycles (350 patients). The researchers found that...
- 1
- 2