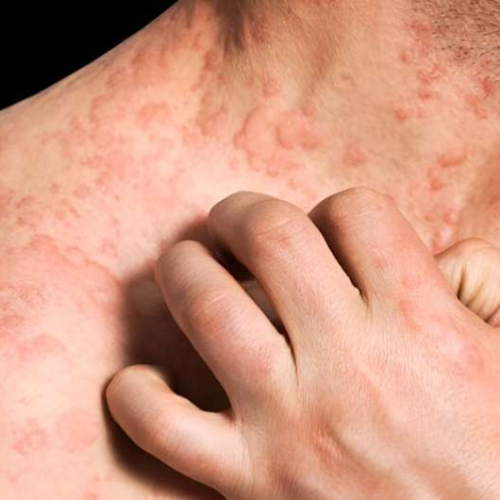
August 16, 2024 The U.S. Food and Drug Administration has approved Galderma’s Nemluvio (nemolizumab) for adult patients living with prurigo nodularis. Nemluvio, administered as a prefilled pen for subcutaneous injection, inhibits interleukin-31 cytokine signaling, which is known to drive itch and is involved in inflammation, altered epidermal differentiation, and fibrosis in prurigo nodularis. The approval...
Tag: <span>prurigo nodularis</span>
Post
Nemolizumab improves symptoms in prurigo nodularis: Clinical trial
by Elana Gotkine For patients with moderate-to-severe prurigo nodularis, nemolizumab improves symptoms, according to a study published in the Oct. 26 issue of the New England Journal of Medicine.Shawn G. Kwatra, M.D., from the Johns Hopkins University School of Medicine in Baltimore, and colleagues conducted a phase 3, double-blind, randomized trial involving adults with moderate-to-severe...