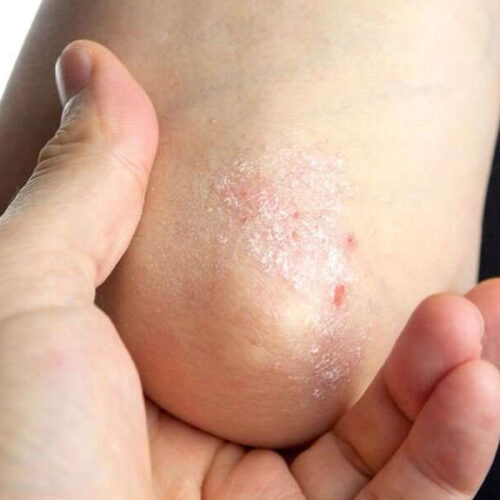
HUNTINGTON BEACH, Calif. — The US Food and Drug Administration (FDA) approvals of tapinarof 1% cream and roflumilast 0.3% cream as treatment options for patients with plaque psoriasis came more than 1 year after the American Academy of Dermatology/National Psoriasis Foundation Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine...
Tag: <span>Tapinarof effective</span>
Post
Tapinarof effective for longer-term treatment of plaque psoriasis
Four in 10 plaque psoriasis patients achieve complete disease clearance with tapinarof cream, 1 percent, according to a study published online June 26 in the Journal of the American Academy of Dermatology. Bruce Strober, M.D., Ph.D., from Yale University in New Haven, Connecticut, and colleagues assessed the long-term safety, efficacy, remittive effect, durability of response, and...